Composition liposome of ascorbyl palmitate and adriamycin
A technology of ascorbyl palmitate and ascorbyl ester, which is applied in the field of compound liposome preparation of ascorbyl palmitate and doxorubicin, can solve the problem of failure to achieve synergistic effect, low content of ascorbyl palmitate, and obvious Doxorubicin particles and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0110] Embodiment 1: a kind of composition liposome formulation of compound ascorbyl palmitate / doxorubicin and its preparation are as follows:
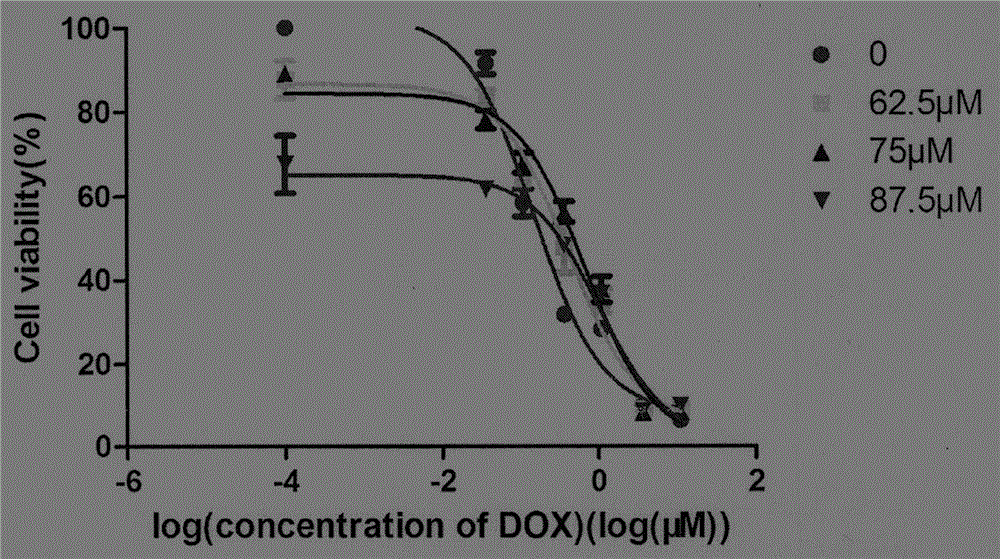
[0111] Experimental method: The liposome preparation process of the compound ascorbyl palmitate / doxorubicin is: dissolving doxorubicin, ascorbyl palmitate, dimyristate phosphatidylcholine, and cholesterol in 15 mL of chloroform , vortexed until completely dissolved, then added to a 500mL eggplant-shaped flask, evaporated under reduced pressure at 70°C to remove the organic solvent, and prepared a uniform lipid film on the wall of the bottle, dried overnight at 75°C in a vacuum oven, then added 15mL of distilled water, after the lipid film was washed off by ultrasonication, the probe was ultrasonicated for 2 cycles in an ice-water bath (30%, 20s, ultrasonication for 1s and off for 1s).
[0112] Liposome evaluation method:
[0113] The measurement of the particle diameter of liposome: adopt laser particle size analyzer to measure.
[...
Embodiment 2
[0122] Embodiment 2: a kind of composition liposome formulation of compound ascorbyl palmitate / doxorubicin and its preparation are as follows:
[0123] Experimental method: the liposome preparation process of the compound ascorbyl palmitate / doxorubicin is as follows: adriamycin, ascorbyl palmitate, soybean lecithin, and sitosterol are dissolved in 15mL of chloroform, vortexed to complete After dissolving, add it to a 500mL eggplant-shaped flask, remove the organic solvent by rotary decompression evaporation at 60°C, and obtain a uniform lipid film on the wall of the bottle, place it in a vacuum drying oven at 35°C to dry overnight, add 15mL of distilled water, and ultrasonically After the lipid film was washed off, the probe was ultrasonicated for 4 cycles in an ice-water bath (80%, 50 s, ultrasonic 1 s off 1 s), and the product was obtained.
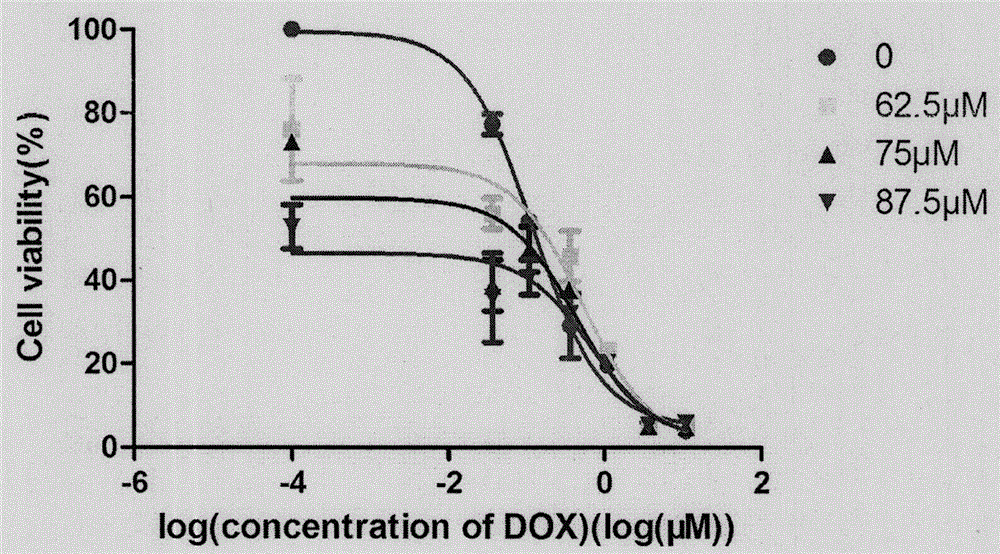
[0124] Experimental results: see Table 2.
[0125] Table 2. The experimental result of embodiment 2
[0126]
[0127] Doxorubicin...
Embodiment 3
[0129] Embodiment 3: a kind of composition liposome formulation of compound ascorbyl palmitate / doxorubicin and its preparation are as follows:
[0130] Experimental method: the liposome preparation process of the compound ascorbyl palmitate / doxorubicin is: dissolving doxorubicin, ascorbyl palmitate, dipalmitate phosphatidylcholine, cholesterol hemisuccinate in 15mL three In methyl chloride, vortex until completely dissolved, then add to a 500mL eggplant-shaped flask, remove the organic solvent by rotary evaporation under reduced pressure at 70°C, prepare a uniform lipid film on the bottle wall, and dry in a vacuum oven at 75°C overnight Finally, add 15mL of distilled water, ultrasonically wash off the lipid film, and perform 6 cycles of ultrasound with the probe in an ice-water bath (30%, 60s, ultrasonication for 1s and stop for 1s).
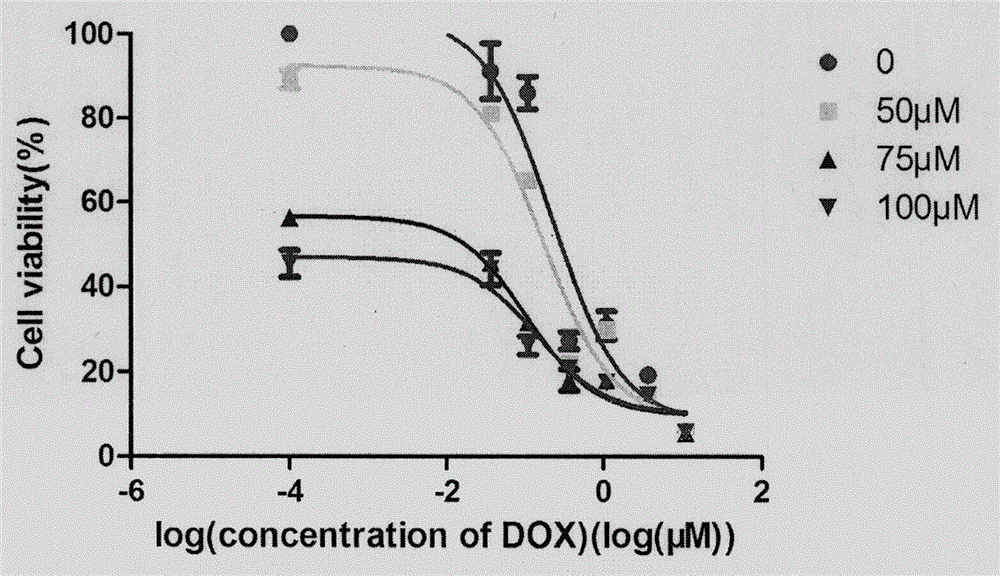
[0131] Experimental results: see Table 3.
[0132] Table 3. The experimental result of embodiment 3
[0133]
[0134] Doxorubicin: DOX, As...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com