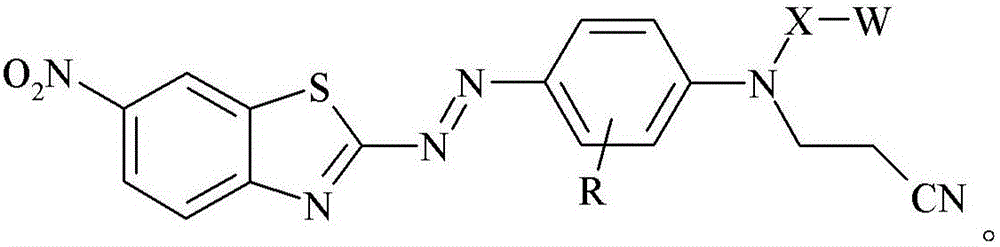
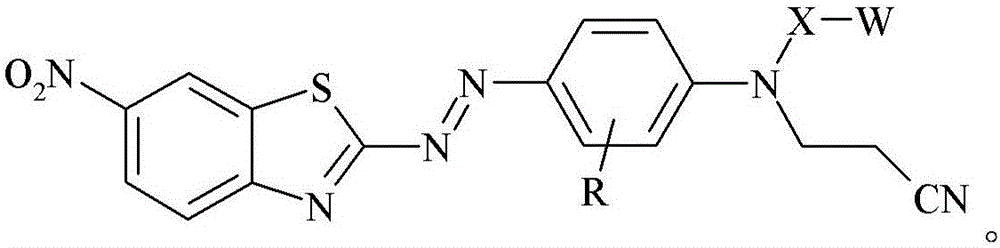
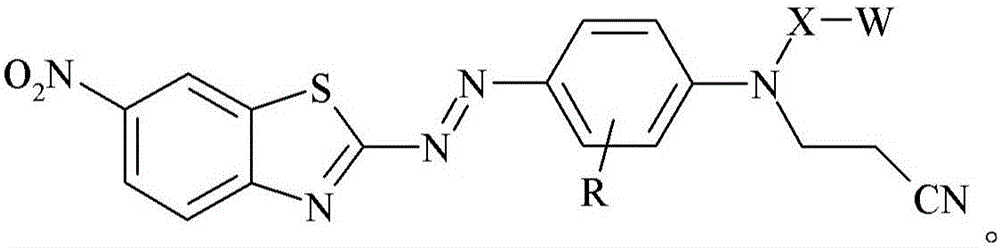
Dye, filler made therefrom, compositions including the filler, and method of determining degree of cure of such compositions
A composition and compound technology, applied in chemical instruments and methods, dyeing low-molecular organic compound treatment, organic dyes, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0185]0.9759 grams of glass bubbles obtained from 3M Company (St. Paul, Minnesota) under the trade designation "GLASS BUBBLES S15" were added to a 100 mL round bottom flask containing 20 mL of deionized water, and the suspended The solution was stirred at 21 °C for 10 minutes. The glass bubbles were then isolated by vacuum filtration and washed with 10 mL of deionized water followed by 50 mL of ethanol. 1 mL of [3-(triethoxysilyl)propyl]-carbamic acid 2-{(2-cyanoethyl)-[4-(6-nitrobenzothiazol-2-ylazo)- Phenyl]amino}ethyl ester was added to a 20 mL glass vial, followed by 5 mL of 95% by volume ethanol in water and incubated at 21 °C in a model "WRISTACTION SHAKER MODEL 75" (Burrell Scientific, Pittsburgh, PA). (Burrell Scientific (Pittsburgh, Pennsylvania)) was mixed on a mechanical shaker for 5 minutes. The vial was removed from the shaker, the washed glass bubbles added, and the vial returned to the shaker for 2 hours. The resulting stained glass bubbles were then isolated...
Embodiment 2
[0187] 3-[4-(2-{(2-cyanoethyl)-[4-(6-nitro-benzothiazol-2-ylazo)-phenyl]-amino}-ethoxy)-benzene A 2.1 mg / mL solution of propionic acid in acetone was prepared by adding 8.2 mg of 3-[4-(2-{(2-cyanoethyl)-[4-(6-nitro-benzothiazole-2 -[-[[-[[]-[[-]-azo)-phenyl]-amino}-ethoxy)-phenyl]-propionic acid was prepared by dissolving in 4.0 mL of acetone. 0.3020 grams of ultra-fine uncoated calcium carbonate obtained from Solvay Chemicals, Inc. (Brussels, Belgium) under the trade designation "SOCAL 31" was added to a 20 mL vial in which containing 3-[4-(2-{(2-cyanoethyl)-[4-(6-nitro-benzothiazol-2-ylazo)-phenyl]-amino}-ethyl A solution prepared by adding a 2.1 mg / mL solution of oxy)-phenyl]-propionic acid in acetone to 1.7 mL of acetone. The vial was capped and the contents mixed on a mechanical shaker model "WRIST ACTIONSHAKER MODEL 75" (Burrell Scientific (Pittsburgh, Pennsylvania)) at about 21°C 2 hours. The resulting stained calcium carbonate was isolated by vacuum filtration and ...
Embodiment 3
[0189] 3-[4-(2-{(2-cyanoethyl)-[4-(6-nitro-benzothiazol-2-ylazo)-phenyl]-amino}-ethoxy)-benzene A 2.1 mg / mL solution of propionic acid in acetone was prepared by adding 8.2 mg of 3-[4-(2-{(2-cyanoethyl)-[4-(6-nitro-benzothiazole-2 -[-[[-[[]-[[-]-azo)-phenyl]-amino}-ethoxy)-phenyl]-propionic acid was prepared by dissolving in 4.0 mL of acetone. 0.2997 grams of granular sodium metaborate hydrate obtained from Sigma-Aldrich, Company (St. Louis, MO) in St. Louis, Missouri, was added to a 20 mL vial containing 3-[4-(2-{(2-cyanoethyl)-[4-(6-nitro-benzothiazol-2-ylazo)-phenyl]-amino}-ethoxy)- A solution prepared by adding 2.1 mg / mL of phenyl]-propionic acid in acetone to 1.4 mL of acetone. The vial was capped and the contents mixed on a mechanical shaker model "WRIST ACTION SHAKER MODEL 75" (Burrell Scientific (Pittsburgh, Pennsylvania)) at about 21°C 2 hours. The resulting stained sodium metaborate was isolated by vacuum filtration and washed with sufficient acetone until the el...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com