Preparation and application of pH-response type rhodamine grafted lignin-based fluorochrome
A fluorescent dye, lignin-based technology is applied in the field of preparation of rhodamine grafted lignin-based fluorescent dyes, which can solve the problems of fluorescence weakening, quenching, and limiting the application range of materials, and achieve good biocompatibility and excellent water solubility. the effect of good cell permeability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
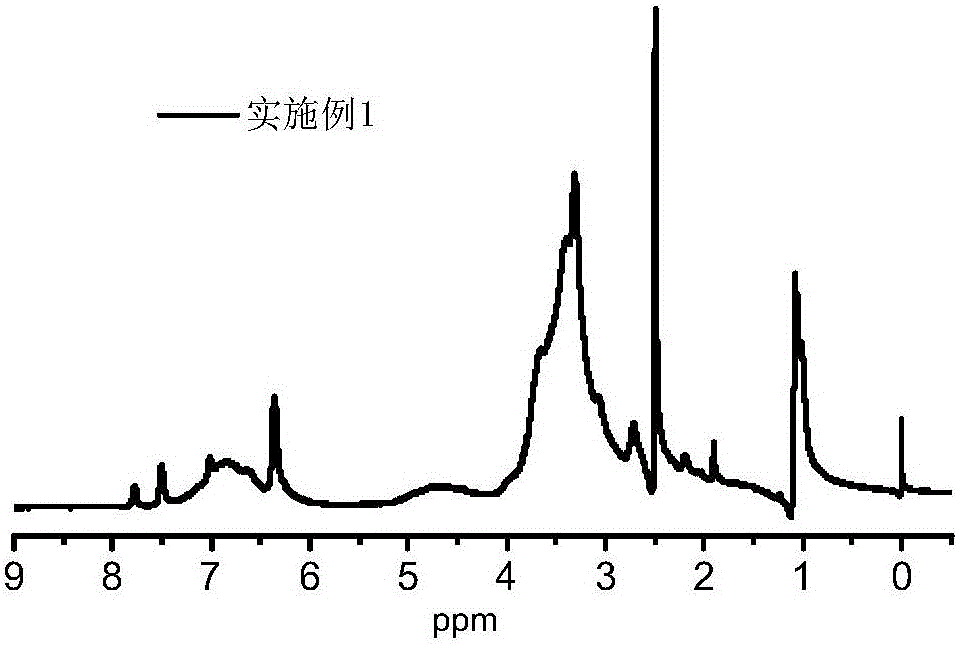
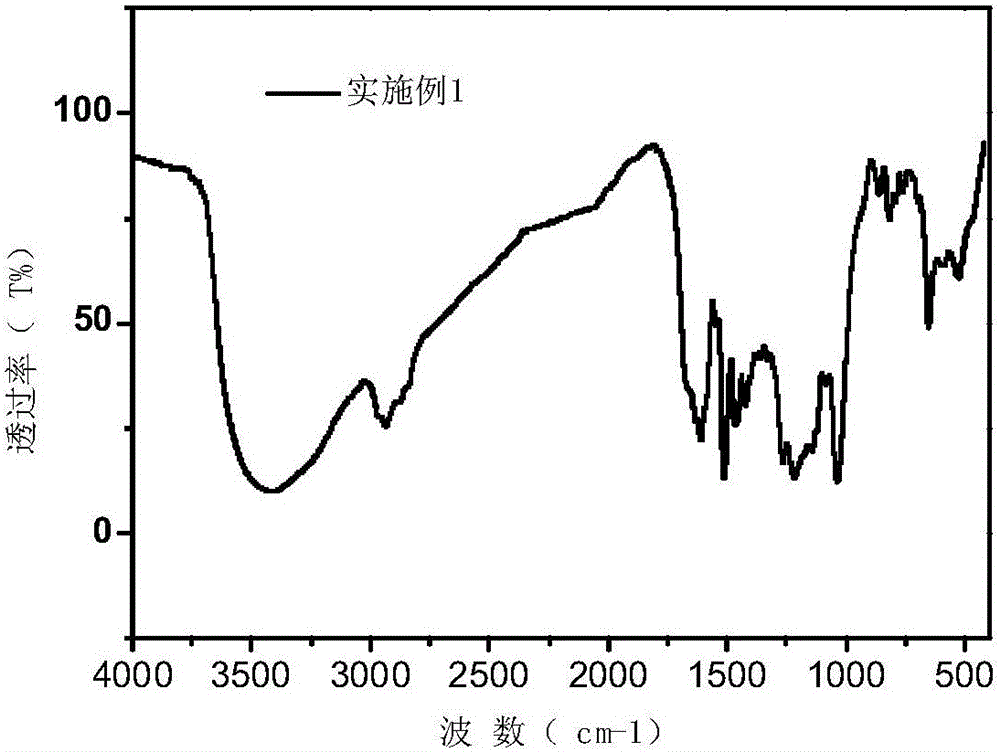
Embodiment 1
[0038] Dissolve 1 g of rhodamine and 4.5 mL of diethylenetriamine in anhydrous methanol, and react at 60° C. for 8 h under the protection of nitrogen. The reaction product is separated and purified to obtain 1.3 g of rhodamine amine derivatives. Dissolve 10g of alkali lignin in 40mL of water, add 2g of NaOH, adjust the pH to 12, and add 0.5g of epichlorohydrin dropwise at the same time, react for 8 hours under normal pressure and heating at 50°C; after that, add 0.5g of NaOH to keep the reaction The pH of the system was 12, the tetrahydrofuran solution of rhodamine amine derivatives (0.2g) was added dropwise, and reacted for 8h under heating conditions at 80°C, then extracted with ethyl acetate to remove unreacted rhodamine derivatives, and finally added to Add 1g of 1,4-butyl sultone and 2g of NaOH to the mixed solution after reaction, heat the reaction at 70°C for 8h, cool to room temperature after the reaction is completed, and then purify through 1000 molecular weight dialy...
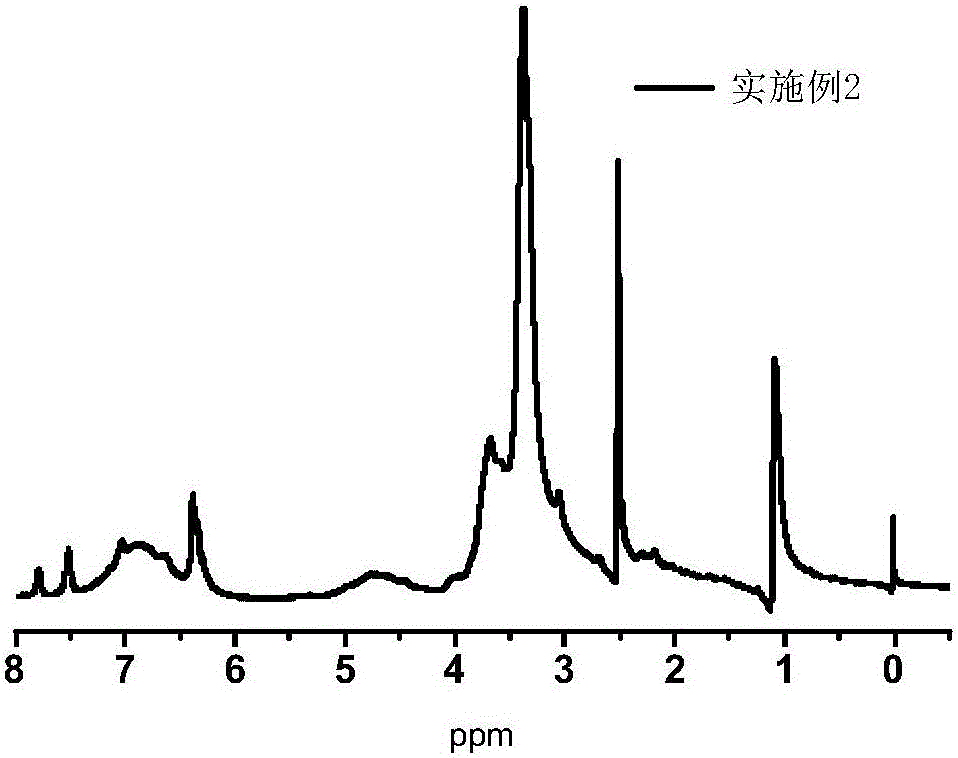
Embodiment 2
[0042] Dissolve 1 g of rhodamine and 2.5 mL of diethylenetriamine in anhydrous methanol, and react for 10 h at 70°C under nitrogen protection. The reaction product is separated and purified to obtain 1.2 g of rhodamine amine derivatives. Dissolve 10g of alkali lignin in 40mL of water, add 4g of NaOH, adjust the pH to 12, and add 1.5g of epichlorohydrin dropwise at the same time, react for 8 hours under normal pressure and heating at 50°C; after that, add 1g of NaOH to maintain the reaction system When the pH is 12, add the tetrahydrofuran solution of the synthesized rhodamine amine derivative (1g) dropwise, and react for 8h under heating conditions at 80°C, then use ethyl acetate to extract and remove the unreacted rhodamine derivative, and finally to the reaction Add 2 g of 1,4-butyl sultone and 4 g of NaOH to the mixed solution, heat and react at 70°C for 8 hours, cool to room temperature after the reaction, purify with anion and cation resins, and freeze-dry to obtain a pH-r...
Embodiment 3
[0044]Dissolve 1 g of rhodamine and 2.5 mL of tetraethylenepentamine in anhydrous methanol, and react at 70°C for 10 h under nitrogen protection. The reaction product is separated and purified to obtain 1.4 g of rhodamine amine derivatives. Take 10g of alkali lignin and dissolve it in 40mL of a mixed solvent of water and ethanol (water:ethanol=1:9), add 4g of KOH, adjust the pH to 12, and add 2g of epichlorobutane dropwise at the same time, under normal pressure, 50 ℃ for 8 h under heating; after that, add 1 g of KOH to keep the pH of the reaction system at 13, add dropwise the tetrahydrofuran solution of rhodamine amino derivatives (2 g), and react under heating at 80 °C for 8 h, then use ethyl acetate Ester extraction to remove unreacted rhodamine derivatives, and finally add 1g of 1,3-propyl sultone and 2g of KOH to the reacted mixed solution, heat the reaction at 70°C for 8h, cool to room temperature after the reaction, after 1000 molecular weight The rhodamine-grafted lig...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com