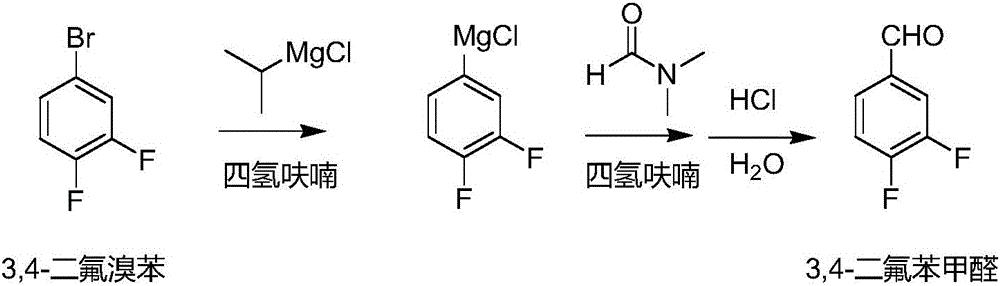
Method for preparing 3, 4-difluorobenzaldehyde
A technology of difluorobenzaldehyde and difluorobromobenzene, which is applied in the field of preparation of 3,4-difluorobenzaldehyde, can solve the problems of increased impurities, inconsistent thinking, and low product yield, and achieve the effect of improving the reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] 5L four-necked bottle, mechanical stirring, nitrogen protection, sequentially add magnesium strips, 400g tetrahydrofuran and iodine particles, then add 41.7g 2-chloropropane at room temperature, trigger after 10min, the maximum temperature is 40°C, drop the temperature at 30-40°C Add the prepared 2-chloropropane solution, drop it in 4.5 hours, keep it warm for 1 hour, and there is basically no magnesium strip. The temperature was lowered, and a solution of 3,4-difluorobromobenzene (654g of 3,4-difluorobromobenzene and 700g of tetrahydrofuran) was added dropwise at 0-10°C, and the addition was completed within 50 minutes. Insulation reaction, up to room temperature. After the reaction was completed, the temperature was lowered, and a DMF solution (291.3 g of DMF and 300 g of tetrahydrofuran) was added dropwise at 0-10° C. for 40 min. Control the temperature below 10°C, add 700g of water dropwise, then add concentrated hydrochloric acid dropwise to adjust the pH to about...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com