Preparation process for propylene from methanol or dimethyl ether
A technology of dimethyl ether and methanol, which is applied in the field of producing propylene from methanol or dimethyl ether, can solve the problems of high cost and high energy consumption, and achieve the effects of increased yield, shortened separation process, and reduced energy consumption for product gas compression and separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
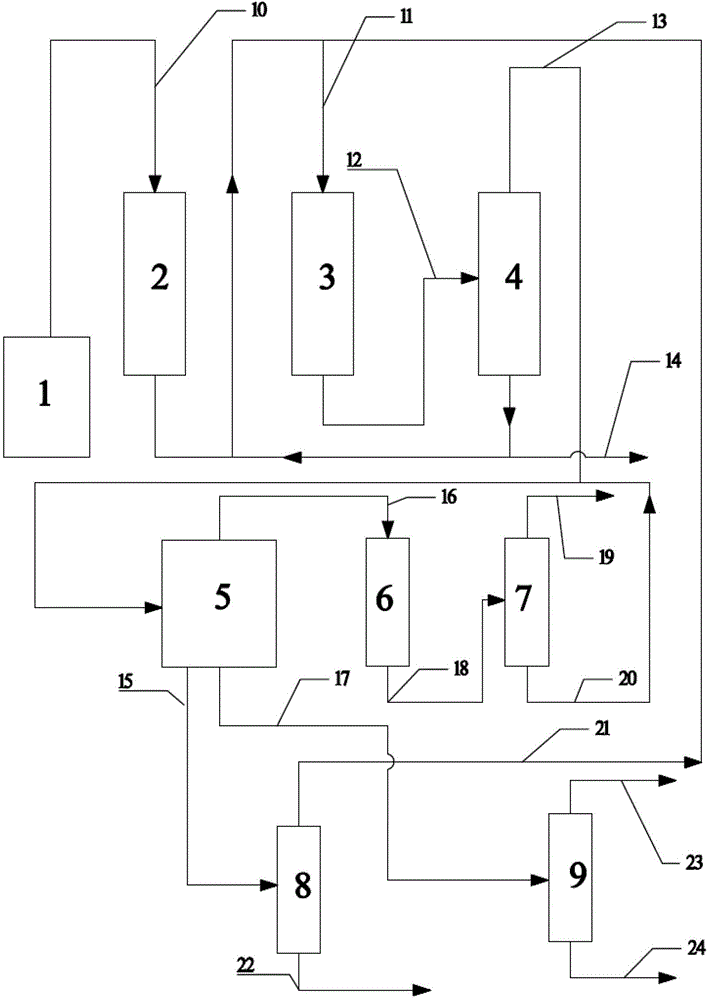
Image
Examples
Embodiment 1
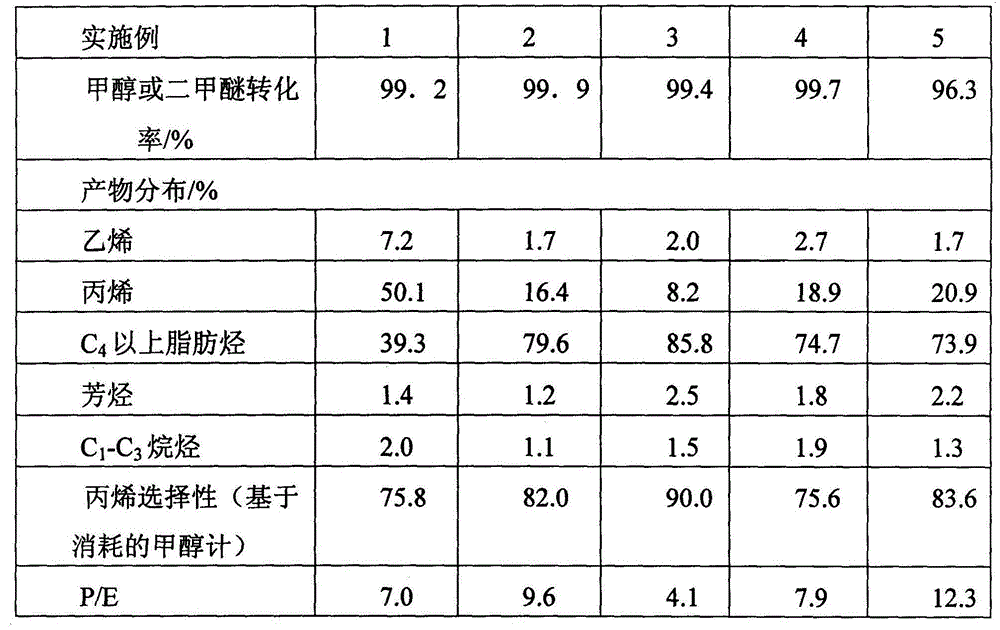
[0040] The first reactor is composed of two adiabatic fixed-bed reactors connected in parallel for switching reaction regeneration. In each adiabatic fixed-bed reactor, high-silicon ZSM-5 is used as the catalyst, the catalyst Si / Al ratio is 50, and the specific surface area is 350m 2 / g, pore volume 0.112ml / g. In an adiabatic fixed-bed reactor, methanol and separation system returns to C 4 The above components (ie C 4 The above cycle hydrocarbons) are converted into reaction gases rich in propylene. The feed composition mass ratio of the first reactor: Methanol (as CH 2 Count): water: C 4 The above cycle hydrocarbons=1:10:0.5, the reaction is based on methanol feed, and the weight hourly space velocity of methanol is 1h -1 , the reactant inlet temperature is 550°C, and the reaction pressure is 0.30MPa. The reaction results of the first reactor are shown in Table 1.
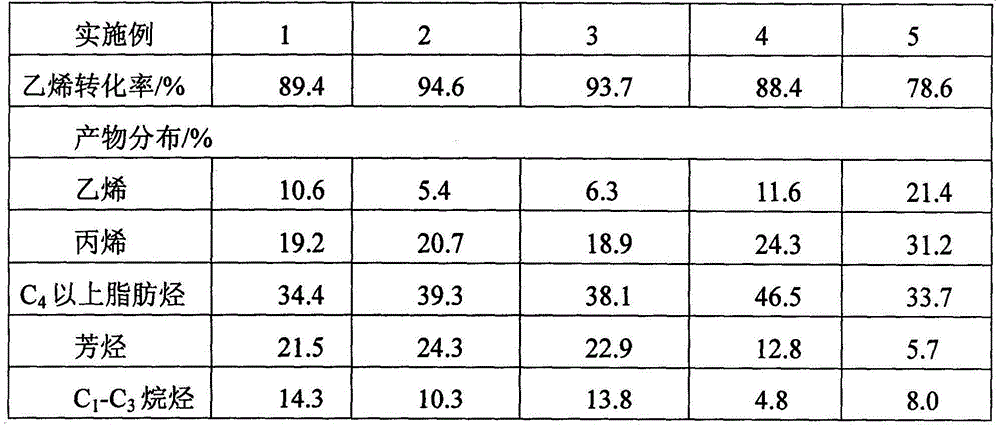
[0041] The second reactor is composed of two adiabatic fixed-bed reactors connected in parallel for switc...
Embodiment 2
[0043] The first reactor consists of five adiabatic fixed-bed reactors connected in parallel, four for reaction and one for catalyst regeneration. In each adiabatic fixed-bed reactor, high-silicon ZSM-5 is used as the catalyst, the catalyst Si / Al ratio is 220, and the specific surface area is 350m 2 / g, pore volume 0.112ml / g. In an adiabatic fixed-bed reactor, methanol and C 4 The above higher hydrocarbons are converted into reaction gases rich in propylene. The feed composition mass ratio of the first reactor: dimethyl ether (as CH 2 Count): water: C 4 The above cycle hydrocarbons=1:5:4, the reaction is based on methanol feed, and the weight hourly space velocity of methanol is 3h -1 , the reactant inlet temperature is 460°C, and the reaction pressure is 0.1MPa. The reaction results of the first reactor are shown in Table 1.
[0044] The second reactor is composed of two nearly isothermal tubular fixed-bed reactors connected in parallel, one for reaction and one for cat...
Embodiment 3
[0046] The first reactor is composed of three adiabatic fixed-bed reactors connected in parallel with cold shock of intermediate raw materials, two for reaction and one for regeneration. In each adiabatic fixed-bed reactor, high-silicon ZSM-5 is used as the catalyst, the catalyst Si / Al ratio is 500, and the specific surface area is 350m 2 / g, pore volume 0.112ml / g. In the reactor, methanol and C 4 The above higher hydrocarbons are converted into reaction gases rich in propylene. The reactant inlet temperature is 380°C, the reaction pressure is 0.13MPa, and the feed composition mass ratio of the first reactor is methanol (in the form of CH 2 Count): water: C 4 The above cycle hydrocarbon = 1:0:10, the reaction is based on methanol feed, and the space velocity is 10h -1 . The reaction results of the first reactor are shown in Table 1.
[0047] The second reactor consists of five nearly isothermal tubular fixed-bed reactors connected in parallel, four for reaction and one f...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com