Hydrogenation method of acryionitrile-butadiene-rubber latex
A technology of nitrile rubber and hydrogenation reaction, which is applied in the field of direct hydrogenation of nitrile latex by using hydrazine derivatives, can solve problems such as damage and mechanical property degradation, reduce pollution, improve processing performance and mechanical properties, and react The effect of low temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
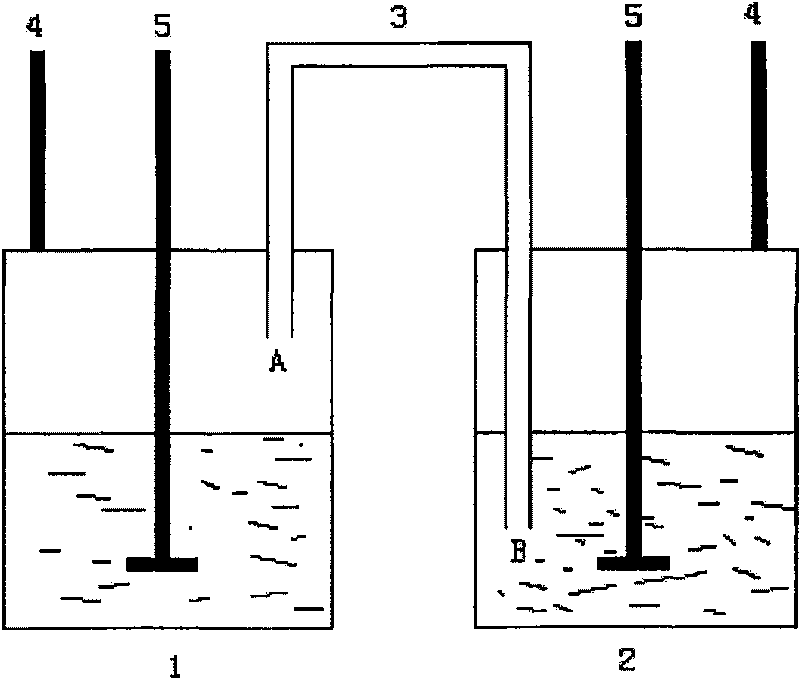
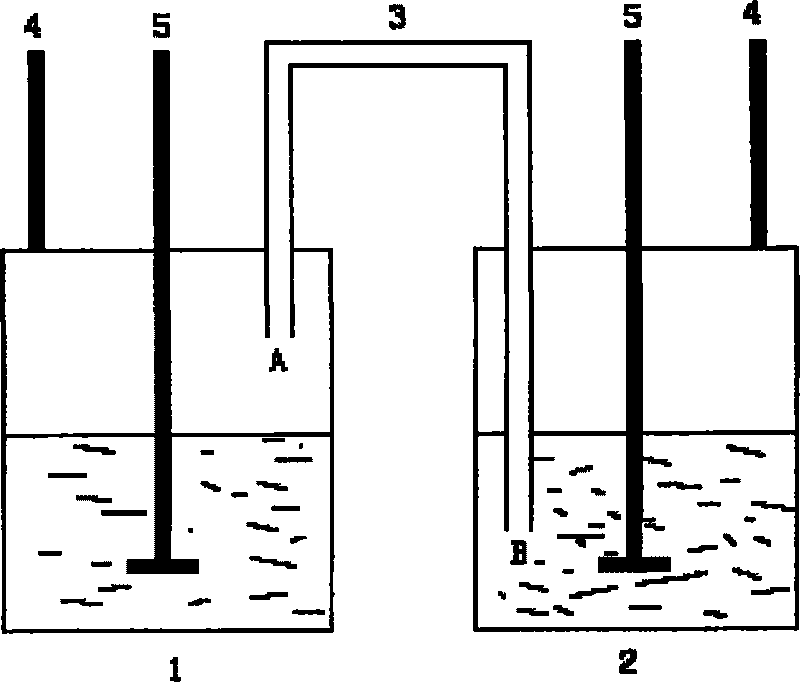
[0031] 1) Add 75ml of xylene into the three-necked bottle 1, extend the end A of the conduit 3 into the three-necked bottle 1, and place it above the liquid level;
[0032] 2) Add 25mL of nitrile latex into the there-necked bottle 2, and add a PTFE rotor, and extend the B end of the conduit 3 below the surface of the nitrile latex;
[0033] 3) Heat the three-necked bottle 1 and the three-necked bottle 2 at the same time. After the three-necked bottle 1 is heated to 130°C and the three-necked bottle 2 is heated to 50°C, react at a constant temperature for 8 hours. During the constant temperature period, add 4g of Toluenesulfonylhydrazide, the total addition of p-toluenesulfonylhydrazide is 32g, and finally the product hydrogenated nitrile latex is obtained.
[0034] The product can be dissolved in chloroform solvent after being demulsified and dried, and the hydrogenation degree is measured to be 35%.
Embodiment 2
[0036] 1) Add 75ml of xylene into the three-necked bottle 1, extend the end A of the conduit 3 into the three-necked bottle 1, and place it above the liquid level;
[0037] 2) Add 25mL of nitrile latex into the there-necked bottle 2, and add a PTFE rotor, and extend the B end of the conduit 3 below the surface of the nitrile latex;
[0038] 3) Heat the three-necked bottle 1 and the three-necked bottle 2 at the same time, after the three-necked bottle 1 is heated to 145°C and the three-necked bottle 2 is heated to 70°C, react at a constant temperature for 48 hours. During the constant temperature period, add 3 g of Toluenesulfonyl hydrazide, the total addition of p-toluenesulfonyl hydrazide is 144g, finally obtains the product hydrogenated nitrile latex.
[0039] The product can be dissolved in chloroform solvent after being demulsified and dried, and the hydrogenation degree is measured to be 39%.
Embodiment 3
[0041] 1) Add 75ml of xylene into the three-necked bottle 1, extend the end A of the conduit 3 into the three-necked bottle 1, and place it above the liquid level;
[0042] 2) Add 25mL of nitrile latex into the there-necked bottle 2, and add a PTFE rotor, and extend the B end of the conduit 3 below the surface of the nitrile latex;
[0043]3) Heat the three-necked bottle 1 and the three-necked bottle 2 at the same time, after the three-necked bottle 1 is heated to 135°C and the three-necked bottle 2 is heated to 60°C, react at a constant temperature for 12 hours. During the constant temperature period, add 3 g of Toluenesulfonyl hydrazide, the total addition of p-toluenesulfonyl hydrazide is 36g, finally the product hydrogenated nitrile latex is obtained.
[0044] The product can be dissolved in chloroform solvent after being demulsified and dried, and the hydrogenation degree is measured to be 65%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com