Dicarbonyl bridged pyrrolo-pyrrole-dione polymer as well as preparation method and application thereof
A polymer, polymerization reaction technology, applied in semiconductor/solid-state device manufacturing, electrical components, circuits, etc., can solve the problem that the mobility cannot meet the wide range of applications, achieve excellent thermal stability, wide UV-Vis absorption spectrum, good film-forming effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
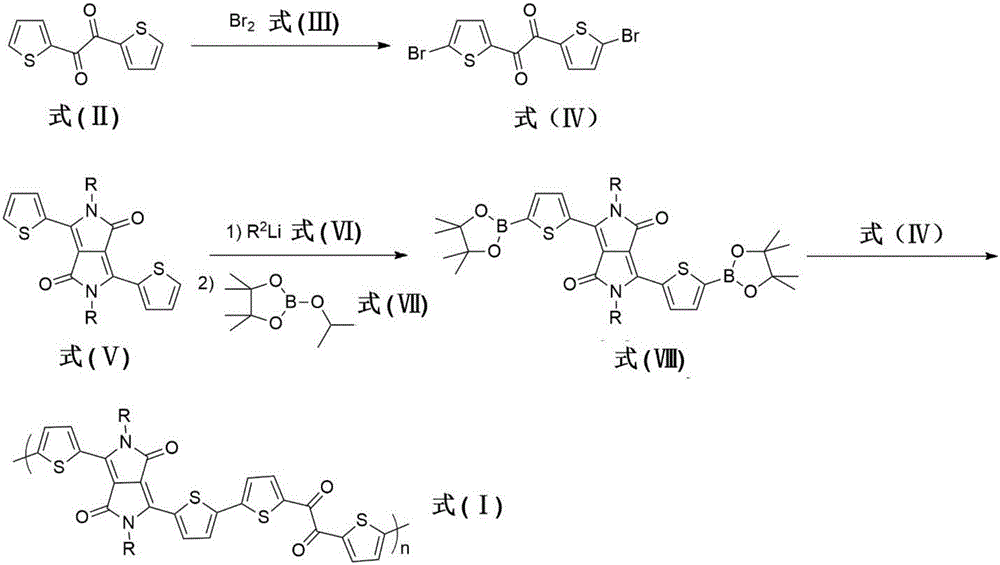
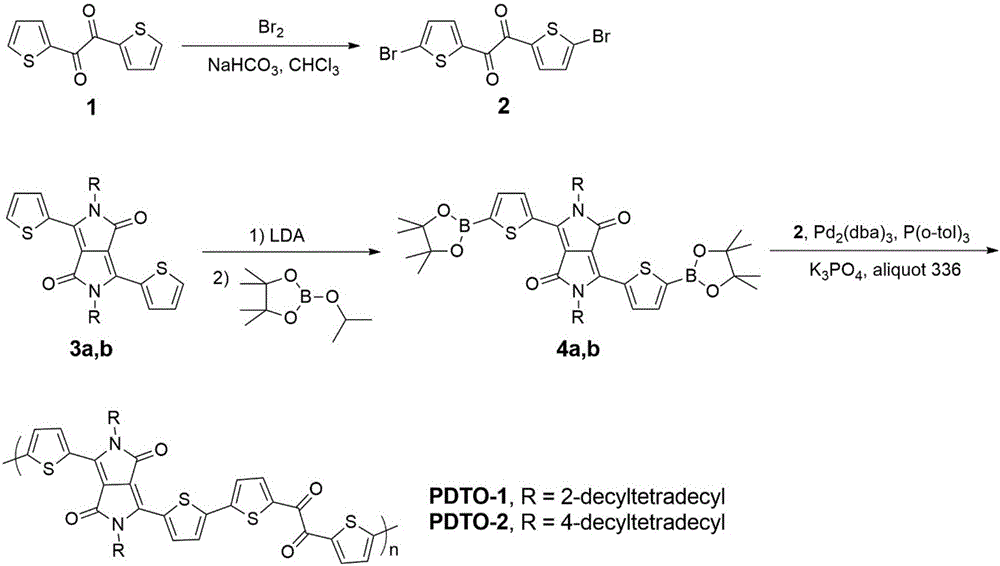
[0042] Example 1, polymer PDTO-1 synthesis (R=2-decyl dodecyl in formula I) (its synthetic route is as follows figure 2 shown)
[0043] 1) Synthesis of compound 1,2-bis(5-bromothienyl)ethane-1,2-dione (2) belonging to formula IV
[0044] Add 1,2-bis(2-thienyl)ethane-1,2-dione (2.0 g, 10 mmol), sodium bicarbonate (3.4 g, 40 mmol) in chloroform (40 mL ) solution was cooled to 0°C and liquid bromine (2.0 ml, 40 mmol) was slowly added dropwise. After completion, the reaction system was heated to 60° C. and stirred for 4 hours under the protection of argon. After cooling to room temperature, it was poured into water and extracted three times with chloroform. After removing the organic solvent under reduced pressure, the resulting solid residue was recrystallized. The obtained yellow solid was dried under vacuum at room temperature to obtain 2.2 g of the target product. Yield: 58%.
[0045] The structural characterization data are as follows:
[0046] Mass Spectrum: HRMS(m / z):...
Embodiment 2
[0059] Example 2, polymer PDTO-2 synthesis (R=4-decyltetradecyl in formula I) (its synthetic route is as follows figure 2 shown)
[0060] 1) Synthesis of compound 2 belonging to formula IV
[0061] The synthesis of compound 2 was carried out with reference to Example 1.
[0062] 1) Synthesis of compound 4b belonging to formula VIII
[0063] 3b (973 mg, 1.0 mmol), 2-isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane (0.6 ml, 3.0 mmol), tetrahydrofuran (20 mL) was cooled to -20°C, lithium diisopropylamide (1.2 mL, 2.4 mmol) was added dropwise under the protection of argon, and then the temperature was slowly raised to 0°C to continue the reaction for 1 h. The reaction was quenched with saturated aqueous ammonium chloride, extracted with dichloromethane, dried over anhydrous sodium sulfate, recrystallized and dried under vacuum to give a red solid (796 mg). Yield: 65%.
[0064] The structural characterization data are as follows:
[0065] Mass Spectrum: HRMS(m / z):[M] + :1...
Embodiment 3
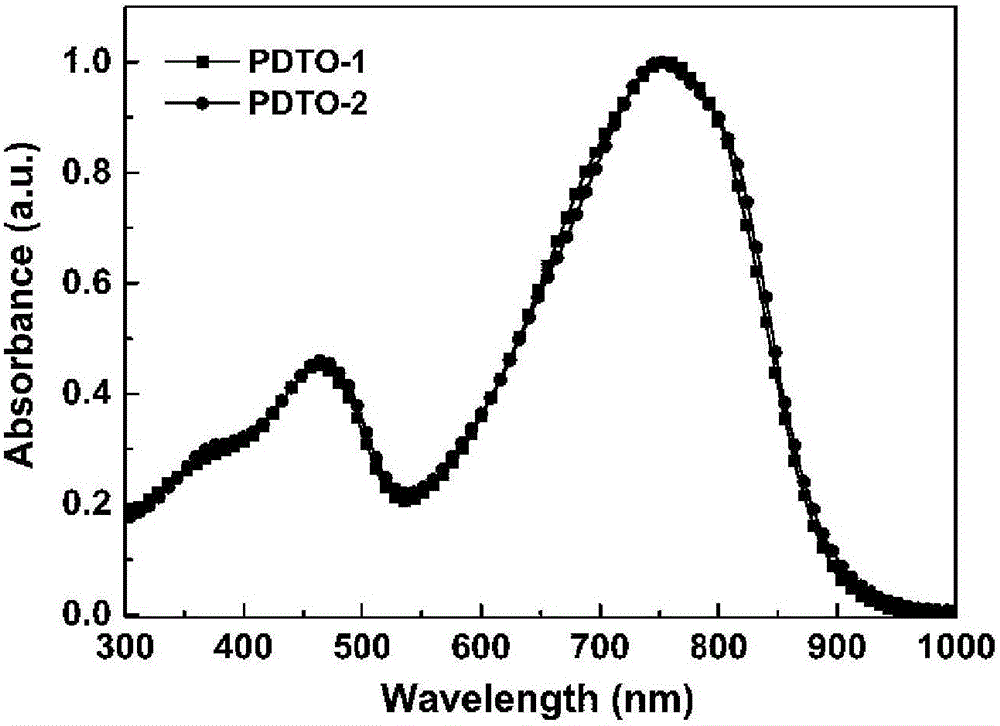
[0073] Spectral properties of embodiment 3, polymer PDTO-1 and PDTO-2
[0074] image 3 and Figure 4 UV-Vis absorption spectra of the polymers PDTO-1 and PDTO-2 chlorobenzene solutions and films prepared for Examples 1 and 2.
[0075] Depend on image 3 It can be seen that this type of polymer exhibits strong absorption in the ultraviolet-visible region and even the near-infrared region, indicating that the polymer molecule has a strong intramolecular charge transfer.
[0076] Depend on Figure 4 It can be seen that the two polymers exhibit strong ordered aggregation or ordered aggregation in the solid.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com