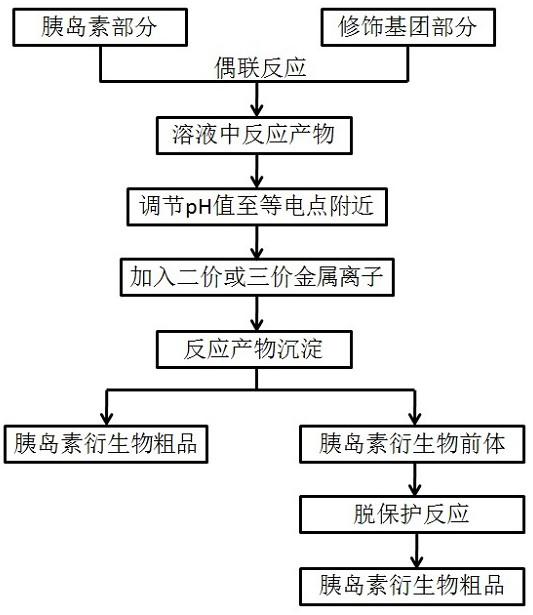
A kind of preparation method of insulin derivative
A technology of insulin derivatives and insulin, which is applied in the field of preparation of insulin derivatives, can solve the problems of loss of reaction products, affecting product yield, and the final yield of insulin derivatives is only 12%.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1 Preparation of insulin degludec without adding metal ions (comparative example).
[0043] The raw material Des(B30)-human insulin was prepared according to the method described in Example 11 of Chinese Patent No. 94193852.2.
[0044] The raw material tert-butylhexadecanedioyl-Glu(OSu)-OtBu was prepared according to the method described in Example 4 of Chinese Patent 200480021733.8.
[0045] Add des(B30) human insulin (500 mg, 0.088 mmol) to 137.5 ml of 50 mM / L NaHCO at 10-15°C 3 In the mixed solution of acetonitrile / water (acetonitrile:water=1:1), add 3.0ml 5% Na 2 CO 3 (W / V) aqueous solution to adjust the solution to clear (pH=10-11). In addition, tert-butylhexadecanedioyl-Glu(OSu)-OtBu (111mg, 0.176mmol) was dissolved in 5ml of acetonitrile, and then added to the above des(B30) human insulin solution at 10-15°C for coupling reaction . React for 1 hour.
[0046] After the coupling reaction, the pH of the reaction solution was adjusted to 6-7 with HCl, t...
Embodiment 2
[0049] Example 2 Adding Zn 2+ Ion preparation insulin degludec (Zn 2+ The molar ratio to insulin is 0.8:1).
[0050] According to the method of Example 1, des(B30) human insulin and tert-butylhexadecanediyl-Glu(OSu)-OtBu were subjected to a coupling reaction.
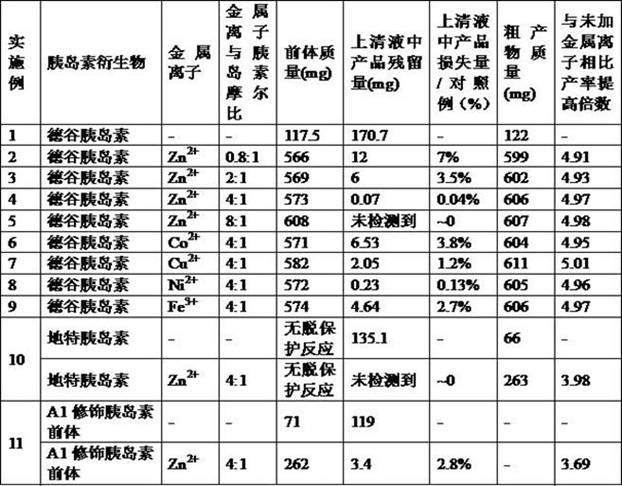
[0051] After the coupling reaction, adjust the pH of the reaction solution to 6-7 with HCl, then add 2 ml ZnCl 2 Aqueous solution (ZnCl 2 At a concentration of 4.8 mg / mL, Zn 2+ The molar ratio of insulin to insulin was 0.8:1) and the precipitate was collected by centrifugation and freeze-dried to obtain 566 mg of crude insulin degludec precursor. The centrifuged supernatant was subjected to chromatographic analysis, and the residual insulin degludec precursor in the supernatant was determined to be 12.0 mg by the external standard method. Compared with Example 1 of the control example, the loss of insulin degludec precursor remaining in the supernatant was 7% (12.0 mg / 170.7 mg) of the control example.
[0052] The...
Embodiment 3
[0054] Example 3 Adding Zn 2+ Ion preparation insulin degludec (Zn 2+ The molar ratio to insulin is 2:1).
[0055] According to the method of Example 1, des(B30) human insulin and tert-butylhexadecanediyl-Glu(OSu)-OtBu were subjected to a coupling reaction.
[0056] After the coupling reaction, adjust the pH of the reaction solution to 6-7 with HCl, then add 2 ml ZnCl 2 Aqueous solution (ZnCl 2 At a concentration of 12 mg / mL, Zn 2+ The molar ratio of insulin to insulin is 2:1), and the precipitate was collected by centrifugation and freeze-dried to obtain 569 mg of crude insulin degludec precursor. The centrifuged supernatant was subjected to chromatographic analysis, and the residual insulin degludec precursor in the supernatant was determined to be 6.0 mg by the external standard method. Compared with Example 1 of the control example, the loss of insulin degludec precursor remaining in the supernatant was 3.5% (6.0 mg / 170.7 mg) of the control example.
[0057] The abov...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com