22-abeo-stigmasterol benzimidazole compound as well as preparation method and application thereof
A technology of benzimidazole and compound, applied in the field of 22-nor-stigmaster benzimidazole compound and its preparation, which can solve the problems of limited application range and high toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
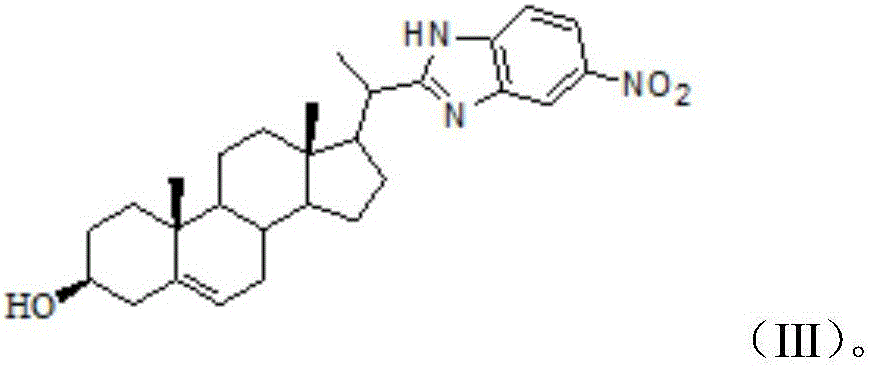
[0024] The present invention provides a kind of 22-nor-stigmaster benzimidazole compound, its structural formula is formula (III):
[0025]
[0026] A preparation method of 22-nor-stigmaster benzimidazole compound, comprising the following steps:
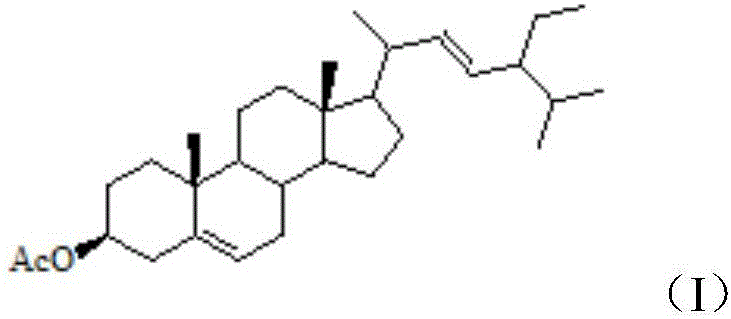
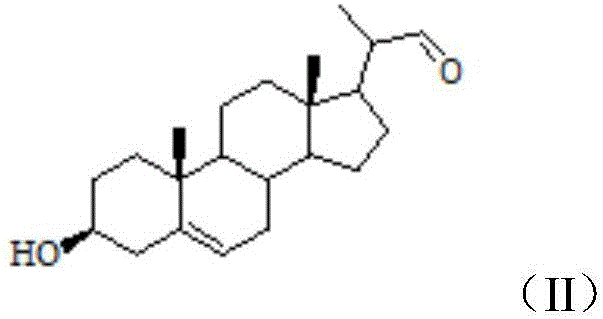
[0027] Step 1, add 12.06g stigmasterol into 10mL pyridine, after completely dissolving, add 5mL acetic anhydride, stir and react at room temperature for 24h, stop the reaction after the reaction is completed, add 30mL dilute hydrochloric acid with a concentration of 1mol / L, and transfer the product into the In the liquid funnel, use 15mL ethyl acetate to continuously extract 3 times (the amount of ethyl acetate is 15mL each time, after adding ethyl acetate for the first time, the solution is layered, and the organic layer is separated after layering, and placed in a conical bottle, then the water layer was extracted with ethyl acetate, after the water layer was extracted, the organic layer was separated, ethyl acetate was added t...
Embodiment 2
[0041] The present invention provides a kind of 22-nor-stigmaster benzimidazole compound, its structural formula is formula (III):
[0042]
[0043] A preparation method of 22-nor-stigmaster benzimidazole compound, comprising the following steps:
[0044] Step 1, add 12.06g stigmasterol into 10mL pyridine, after completely dissolving, add 5mL acetic anhydride, stir and react at room temperature for 24h, stop the reaction after the reaction is completed, add 30mL dilute hydrochloric acid with a concentration of 1mol / L, and transfer the product into the In the liquid funnel, use 15mL ethyl acetate to continuously extract 3 times (the amount of ethyl acetate is 15mL each time, after adding ethyl acetate for the first time, the solution is layered, and the organic layer is separated after layering, and placed in a conical bottle, then the water layer was extracted with ethyl acetate, after the water layer was extracted, the organic layer was separated, ethyl acetate was added t...
Embodiment 3
[0058] The present invention provides a kind of 22-nor-stigmaster benzimidazole compound, its structural formula is formula (III):
[0059]
[0060] A preparation method of 22-nor-stigmaster benzimidazole compound, comprising the following steps:
[0061] Step 1, add 12.06g stigmasterol into 10mL pyridine, after completely dissolving, add 5mL acetic anhydride, stir and react at room temperature for 24h, stop the reaction after the reaction is completed, add 30mL dilute hydrochloric acid with a concentration of 1mol / L, and transfer the product into the In the liquid funnel, use 15mL ethyl acetate to continuously extract 3 times (the amount of ethyl acetate is 15mL each time, after adding ethyl acetate for the first time, the solution is layered, and the organic layer is separated after layering, and placed in a conical bottle, then the water layer was extracted with ethyl acetate, after the water layer was extracted, the organic layer was separated, ethyl acetate was added t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com