A screening method for antithrombotic drugs based on magnetic bead separation
A technology of antithrombotic drugs and screening methods, which is applied in the field of drug screening, can solve the problems of membrane materials, proteins and compounds affecting the accuracy of experiments, poor compatibility between elution solvents and mass spectrometry, and retention of unbound compounds. Short screening time, simple and economical equipment, good reproducible results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1 Preparation of thrombin-bonded magnetic beads
[0027] Take a certain volume of magnetic beads, use an equal volume of 25mM MES solution (pH 6) to wash twice, twice for 10min, add an equal volume of freshly prepared EDC solution and NHS solution (50mg / ml) to the washed magnetic beads , mix well, and incubate at room temperature for 30 min with slow shaking. After incubation, the tube was placed on the magnet for 4 minutes, the supernatant was removed, and finally washed twice with 25 mM MES solution (pH 6) to obtain activated magnetic beads for later use.
[0028] Take 25, 50, 75, and 100 μL of activated magnetic beads respectively, add 200 μL of thrombin (1 mg / ml) solution, shake and mix well, and incubate overnight at room temperature. After incubation, place the tube on the magnetic beads for 4 minutes, remove the supernatant remaining from the binding, and use the Bradford method to determine the protein content in it. Add 100 μL of 0.5% BSA solution to ...
Embodiment 2
[0035] Example 2 Research on various factors of antithrombotic drug screening method based on magnetic bead separation
[0036] (1) Using scutellarin as a model drug, optimize the denaturation and elution solvent
[0037] Take 10 μL of thrombin-bonded magnetic beads prepared in Example 1 above, add 20 μL of scutellarin reference solution (0.1 mg / ml) and 170 μL of PBS buffer (pH 7.4) and mix, and incubate for 30 min. After the incubation, use 200 μL PBS buffer was washed 4 times, and the eluate was analyzed by liquid phase. At last, the volume concentration was 10%, 30%, 50%, 70% and 90% acetonitrile-water solution (v / v) and volume concentration 10%, 30% respectively. %, 50%, 70% and 90% methanol-water solution (v / v) for denaturation elution, and the denaturation eluents were respectively taken for liquid phase analysis.
[0038] (2) Using scutellarin as a model drug, optimize the incubation time
[0039] Take 10 μL of thrombin-bonded magnetic beads prepared in Example 1 above,...
Embodiment 3
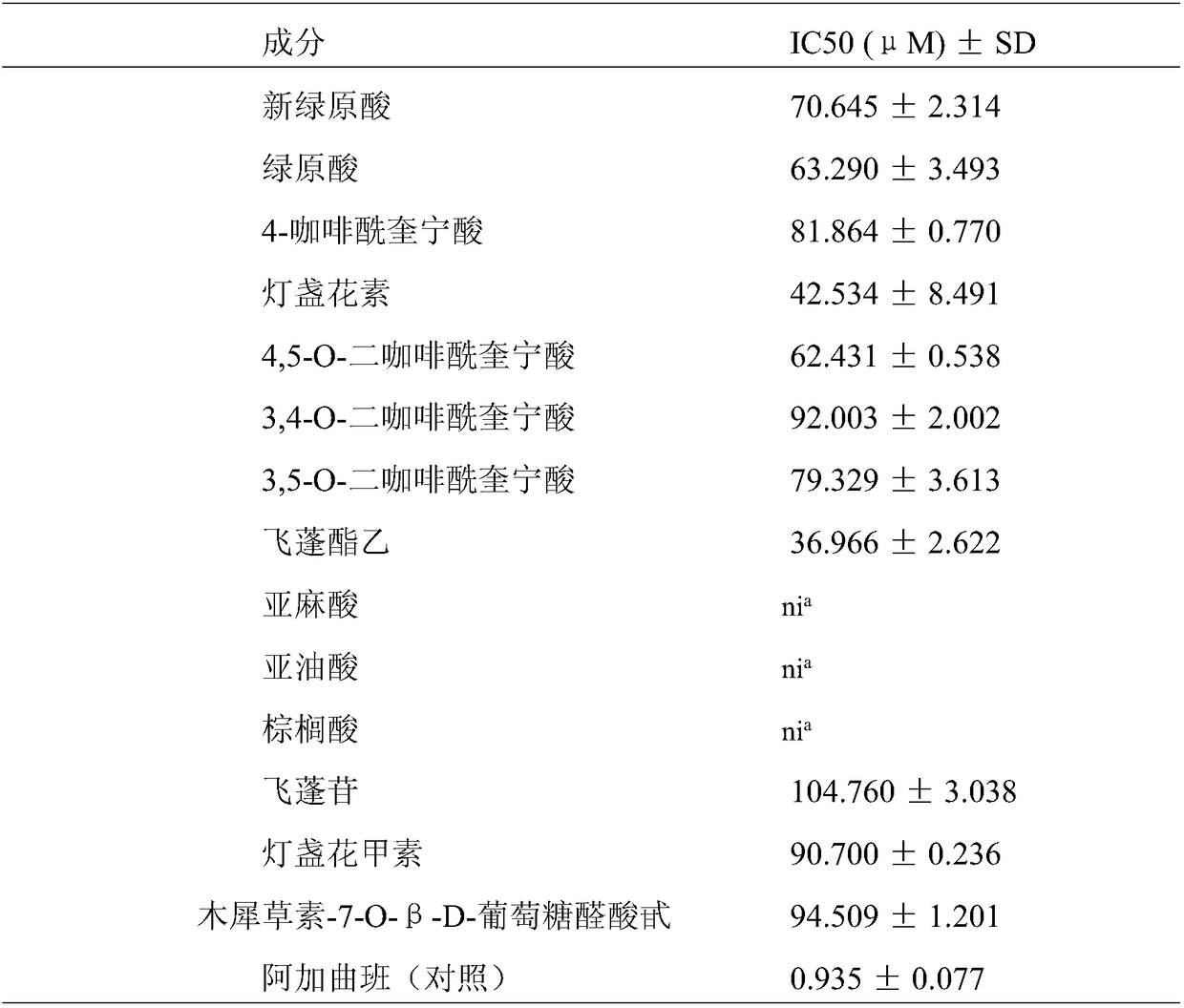
[0053] Example 3 Screening of Antithrombotic Components of Erigeron breviscapine
[0054] (1) Take a certain volume of magnetic beads, wash them twice with an equal volume of 25mM MES buffer solution, each time for 10min, after washing, add an equal volume of 50mg / ml EDC solution and 50mg / ml NHS solution, mix well, and store at room temperature Incubate with slow shaking, after incubation, place on the magnet for 4min, remove the supernatant, and then wash with 25mM MES buffer solution to obtain activated magnetic beads, set aside;
[0055] (2) Take the activated magnetic beads obtained in step (1), add thrombin solution, shake and mix evenly, incubate at room temperature, after incubation, place on the magnetic beads for 4 minutes, remove the remaining supernatant from bonding, and obtain coagulation Enzyme-bonded magnetic beads;
[0056] (3) Weigh 1g of Erigeron breviscapine powder, add 50ml of methanol, reflux for 2h, filter, centrifuge the filtrate at 13400rpm for 10min, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| control rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com