Sodium ilaprazole powder injection and preparation method thereof
A technology of ilaprazole sodium and powder injection, which is applied in the field of ilaprazole sodium powder injection and its preparation, and can solve problems such as allergic reactions and accelerated instability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
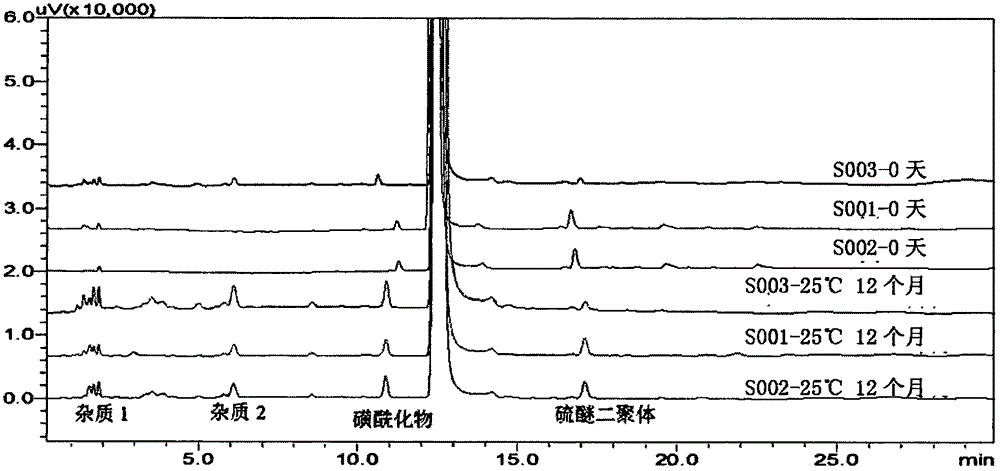
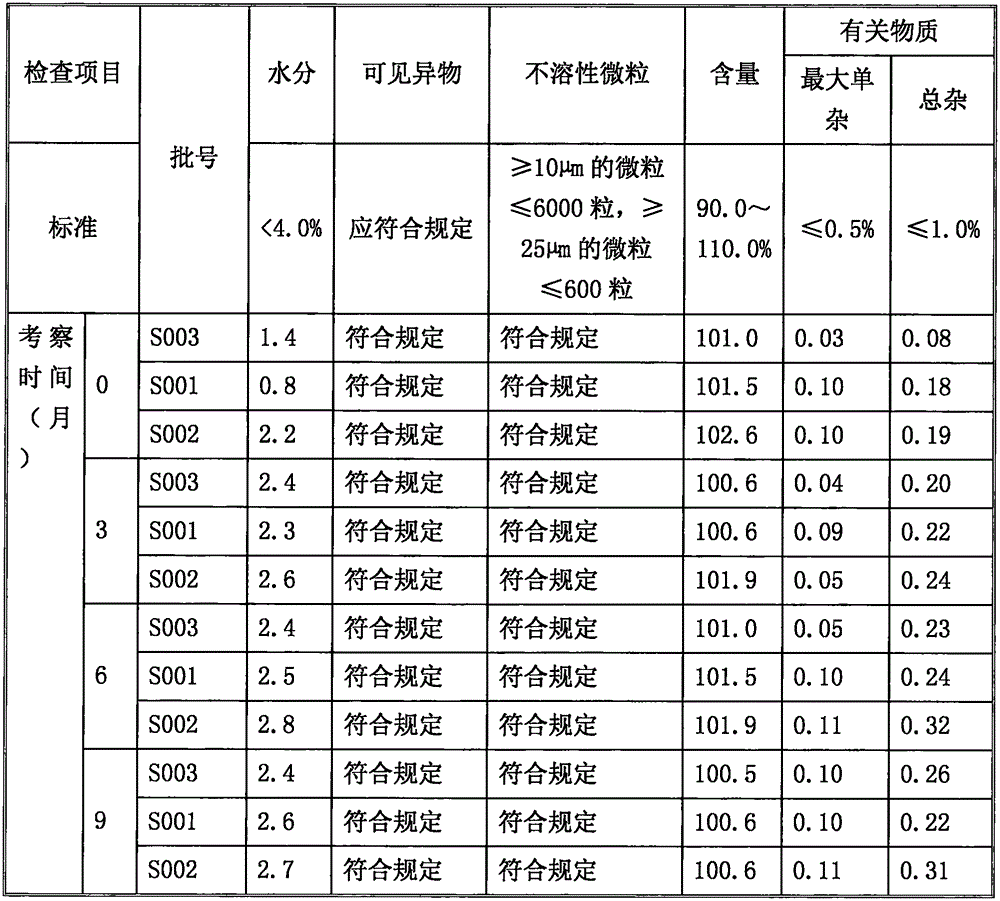
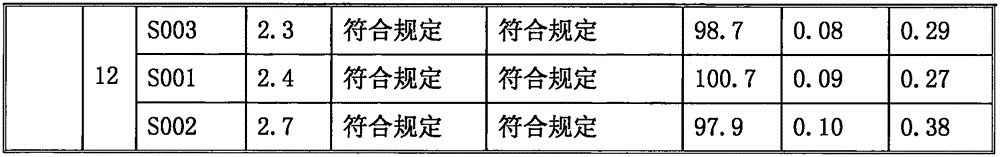
[0031] Embodiment 1: Preparation of ilaprazole sodium freeze-dried powder injection (batch number S001)
[0032] The present embodiment is to adopt the method of the present invention to prepare 1000 bottles of ilaprazole sodium freeze-dried powder injection (marked amount: containing 10 mg ilaprazole sodium, edetate disodium content 0.05%, mannitol content 2.4%), specifically The process consists of the following steps:
[0033] 1) Pre-cool 900ml of water for injection to below 25°C.
[0034] 2) First weigh about 800ml of water for injection, add 0.5g of edetate disodium and 24g of mannitol, and then adjust the pH to 11.5-12.0 with 5mol / L sodium hydroxide. Add 10g of ilaprazole sodium crude drug, fully dissolve, add water for injection to 1000g, and then adjust the pH to 11.5-12.0 with 5mol / L sodium hydroxide. Close the lid of the ingredient jar after dissolving.
[0035] 3) The solution is filtered through two 0.2um filter membranes to the batching room.
[0036] 4) Use ...
Embodiment 2
[0038] Embodiment 2: Preparation of ilaprazole sodium freeze-dried powder injection (lot number S002)
[0039]The present embodiment adopts the method of the present invention to prepare 1000 bottles of ilaprazole sodium freeze-dried powder injection (marked amount: containing 10 mg ilaprazole sodium, edetate disodium content 0.15%, mannitol content 4.6%), specifically The process consists of the following steps
[0040] 1) Pre-cool 900ml of water for injection to below 25°C.
[0041] 2) First weigh about 800ml of water for injection, add 1.5g of edetate disodium and 46g of mannitol, and then adjust the pH to 11.5-12.0 with 5mol / L sodium hydroxide. Add 10g of ilaprazole sodium crude drug, fully dissolve, add water for injection to 1000g, and then adjust the pH to 11.5-12.0 with 5mol / L sodium hydroxide. Close the lid of the ingredient jar after dissolving.
[0042] 3) The solution is filtered through two 0.2um filter membranes to the batching room.
[0043] 4) Use an automa...
Embodiment 3
[0045] Embodiment 3: the preparation of ilaprazole sodium freeze-dried powder injection (lot number S003)
[0046] This embodiment is to adopt the method of the present invention to prepare 1000 bottles of ilaprazole sodium freeze-dried powder injection (marked amount: containing 10 mg ilaprazole sodium, edetate disodium content 0.10%, mannitol content 2.8%), The specific process includes the following steps
[0047] 1) Pre-cool 900ml of water for injection to below 25°C.
[0048] 2) First weigh about 800ml of water for injection, add 1.0g of edetate disodium and 28g of mannitol, and then adjust the pH to 11.5-12.0 with 5mol / L sodium hydroxide. Add 10g of ilaprazole sodium crude drug, fully dissolve, add water for injection to 1000g, and then adjust the pH to 11.5-12.0 with 5mol / L sodium hydroxide. Close the lid of the ingredient jar after dissolving.
[0049] 3) The solution is filtered through two 0.2um filter membranes to the batching room.
[0050] 4) Use an automatic ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com