Chemical chain hydrogen production composite oxygen carrier with anti-carbon property and preparation method of chemical chain hydrogen production composite oxygen carrier
A chemical chain and anti-carbon deposition technology, applied in chemical instruments and methods, inorganic chemistry, chemical/physical processes, etc., can solve the problems of low hydrogen purity, slow reaction speed, and poor hydrogen production effect, and achieve high reactivity, The process is easy to control and the effect of carbon deposition suppression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
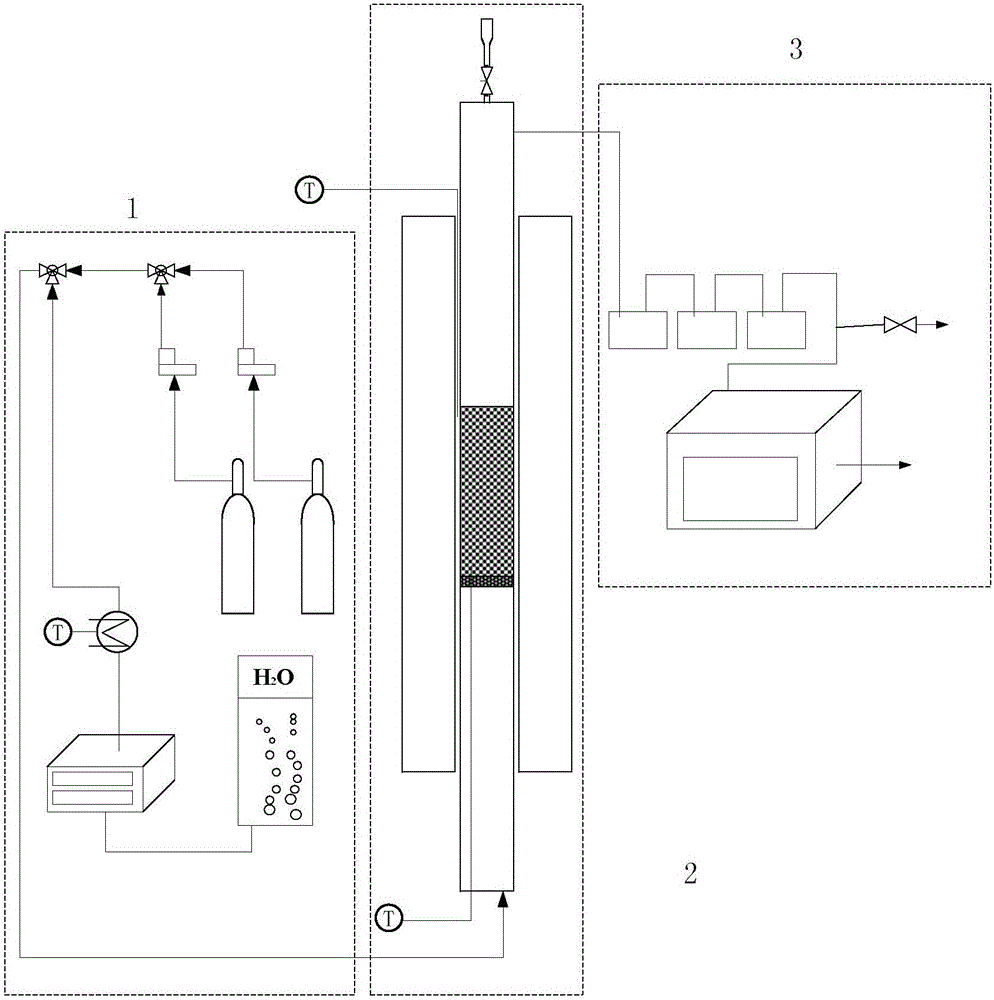
Image
Examples
preparation example Construction
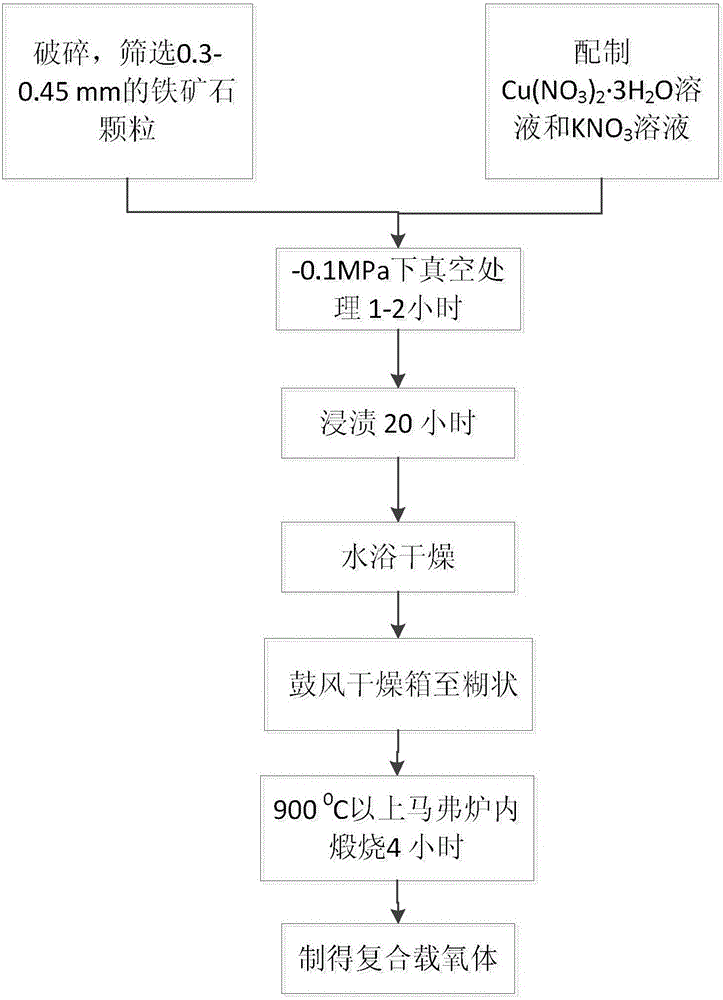
[0030] The preparation method specifically comprises the following steps:
[0031] (1) Crushing and screening: first crush the iron ore with a crusher, and screen out iron ore particles with a particle size range of 0.2-0.45mm;
[0032] (2) Solution preparation: prepare Cu(NO 3 ) 2 ·3H 2 O solution and KNO 3 solution, and stir it with a glass rod to make it evenly mixed;
[0033](3) Vacuum treatment: Add the iron ore obtained in step (1) into a jar with a rubber stopper, use a vacuum pump to vacuumize, and the vacuum degree is -0.1MPa. After a period of time, add the iron ore produced by Continue to vacuumize the mixed solution prepared in step (2) for 1-2 hours;
[0034] (4) Immersion treatment: the suspension obtained in step (3) was sealed and left for 20 hours, and Cu(NO 3 ) 2 ·3H 2 O solution and KNO 3 Impregnation of iron ore by solution;
[0035] (5) Drying treatment: after the mixture obtained in step (4) is dried in a water bath at a water bath temperature o...
Embodiment 1
[0047] The method for preparing a composite oxygen carrier for chemical chain hydrogen production from iron ore specifically includes the following steps:
[0048] (1) Crushing and screening: first, the iron ore is crushed with a crusher, and the iron ore particles with a particle size range of 0.2-0.45 mm are screened out. The main components of the iron ore are shown in Table 1;
[0049] (2) Solution preparation: 50gCu(NO 3 ) 2 ·3H 2 O crystals were dissolved in an appropriate amount of deionized water to prepare Cu(NO 3 ) 2 ·3H 2 O solution. 16.67gKNO 3 The powder is dissolved in an appropriate amount of deionized water to prepare KNO 3 solution. The prepared Cu(NO 3 ) 2 ·3H 2 O solution and KNO 3 Mix the solution and stir it with a glass rod to make it evenly mixed;
[0050] (3) Vacuum treatment: Add 100g of the iron ore obtained in step (1) into a jar with a rubber stopper, and use a vacuum pump to vacuumize the vacuum to -0.1MPa. After a period of time, add ...
Embodiment 2
[0056] The method for preparing a composite oxygen carrier for chemical chain hydrogen production from iron ore specifically includes the following steps:
[0057] (1) Crushing and screening: first, the iron ore is crushed with a crusher, and the iron ore particles with a particle size range of 0.2-0.45 mm are screened out. The main components of the iron ore are shown in Table 1;
[0058] (2) Solution preparation: 48.32gCu(NO 3 ) 2 ·3H 2 O crystals were dissolved in an appropriate amount of deionized water to prepare Cu(NO 3 ) 2 ·3H 2 O solution. 10gKNO 3 The powder is dissolved in an appropriate amount of deionized water to prepare KNO 3 solution. The prepared Cu(NO 3 ) 2 ·3H 2 O solution and KNO 3 Mix the solution and stir it with a glass rod to make it evenly mixed;
[0059] (3) Vacuum treatment: Add 100g of the iron ore obtained in step (1) into a jar with a rubber stopper, and use a vacuum pump to vacuumize the vacuum to -0.1MPa. After a period of time, add ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com