Molecular synthesis method acrylic acid type functional monomer containing supermolecule quadrupolar hydrogen bond structure
A technology of quadruple hydrogen bonds and functional monomers, applied in organic chemistry and other fields, to achieve the effects of easy polymerization, high activity, and simple post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] a. Dissolve 125 mg of 2-amino-4-hydroxy-6-picoline and 310 mg of methacryloyloxyethyl isocyanate in 6 mL of pyridine solution, and react at a temperature of 100 ° C for 16 hours;
[0031] b. Separation and purification of the reaction system obtained in step a: naturally cool the reaction solution to room temperature, a white solid is precipitated, suction filtered, and the filter cake is collected to obtain a white solid, and the white solid is recrystallized by volume ratio of 1:1 chloroform: acetone , and vacuum-dried to obtain a white solid supramolecular quadruple hydrogen-bonded acrylic functional monomer molecule.
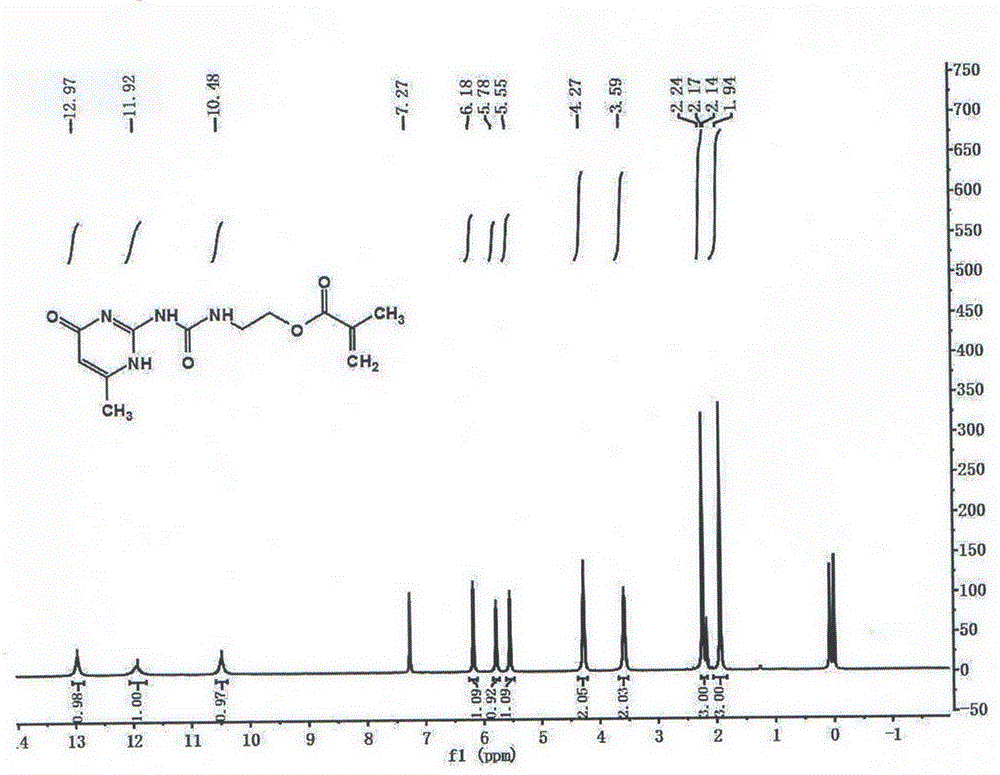
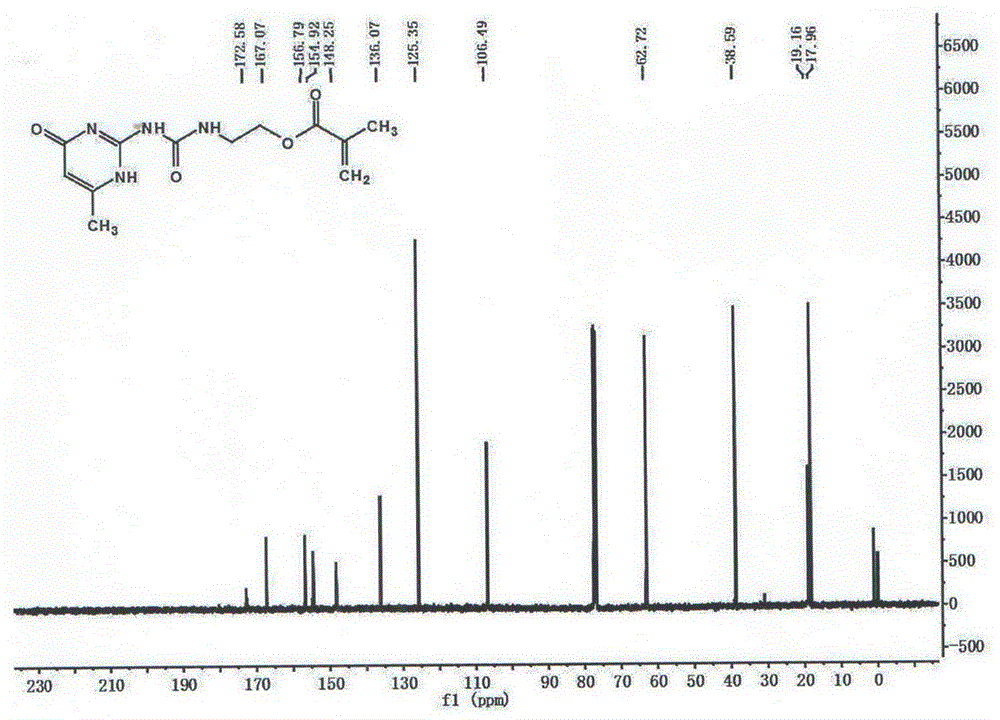
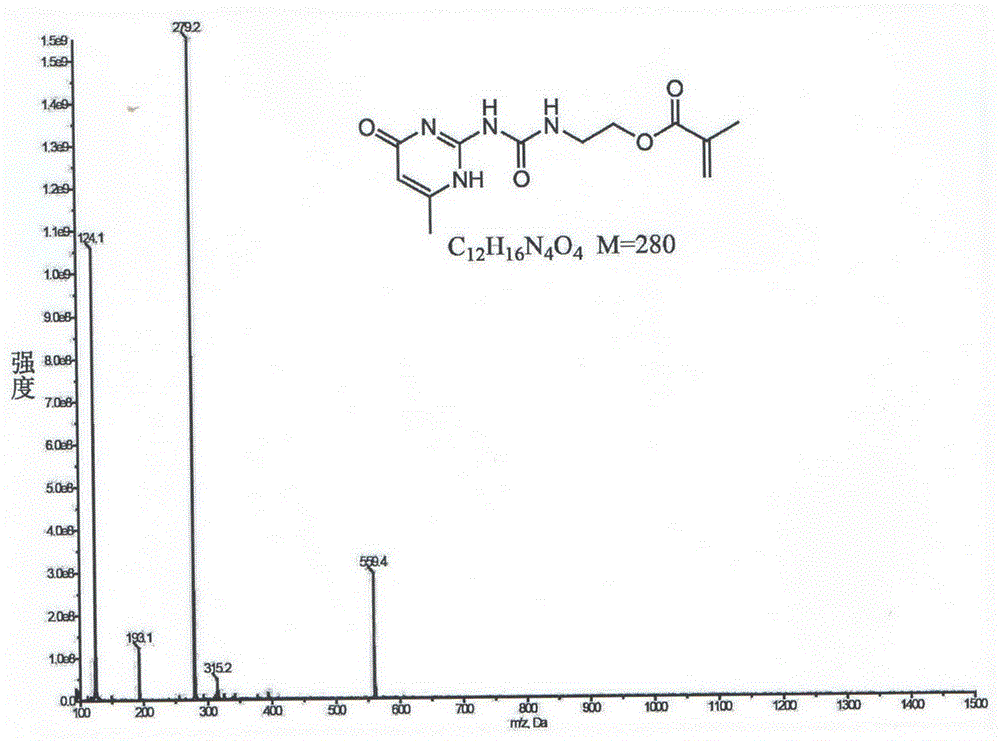
[0032] 1 HNMR (400MHz, CDCl 3 )δ12.97(s,1H),11.92(s,1H),10.48(s,1H),6.18(s,1H),5.78(s,1H),5.55(s,1H),4.27(s,2H ),3.59(s,2H),2.20(d,J=26.8Hz,3H),1.94(s,3H). 13 CNMR (400MHz, CDCl 3 )δ172.58(s),167.30(s),156.79(s),154.92(s),148.25(s),136.07(s),125.78(s),106.49(s),63.09(s),38.80( s),19.16(s),18.30(s).MS(ESI):C 12 h 16 N 4 o 4 M=280.
Embodiment 2
[0034] a. Dissolve 125 mg of 2-amino-4-hydroxy-6-picoline and 388 mg of methacryloyloxyethyl isocyanate in 6 mL of pyridine solution, and react at a temperature of 100 ° C for 16 hours;
[0035] b. Separation and purification of the reaction system obtained in step a: naturally cool the reaction solution to room temperature, a white solid is precipitated, suction filtered, and the filter cake is collected to obtain a white solid, and the white solid is recrystallized by volume ratio of 1:1 chloroform: acetone , and vacuum-dried to obtain a white solid supramolecular quadruple hydrogen-bonded acrylic functional monomer molecule.
[0036] 1 HNMR (400MHz, CDCl 3 )δ12.97(s,1H),11.92(s,1H),10.48(s,1H),6.18(s,1H),5.78(s,1H),5.55(s,1H),4.27(s,2H ),3.59(s,2H),2.20(d,J=26.8Hz,3H),1.94(s,3H). 13 CNMR (400MHz, CDCl 3 )δ172.58(s),167.30(s),156.79(s),154.92(s),148.25(s),136.07(s),125.78(s),106.49(s),63.09(s),38.80( s),19.16(s),18.30(s).MS(ESI):C 12 h 16 N 4 o 4 M=280.
Embodiment 3
[0038] a. Dissolve 125 mg of 2-amino-4-hydroxy-6-picoline and 465 mg of methacryloyloxyethyl isocyanate in 6 mL of pyridine solution, and react at a temperature of 100 ° C for 16 hours;
[0039] b. Separation and purification of the reaction system obtained in step a: naturally cool the reaction solution to room temperature, a white solid is precipitated, suction filtered, and the filter cake is collected to obtain a white solid, and the white solid is recrystallized by volume ratio of 1:1 chloroform: acetone , and vacuum-dried to obtain a white solid supramolecular quadruple hydrogen-bonded acrylic functional monomer molecule.
[0040] 1 HNMR (400MHz, CDCl 3 )δ12.97(s,1H),11.92(s,1H),10.48(s,1H),6.18(s,1H),5.78(s,1H),5.55(s,1H),4.27(s,2H ),3.59(s,2H),2.20(d,J=26.8Hz,3H),1.94(s,3H). 13 CNMR (400MHz, CDCl 3 )δ172.58(s),167.30(s),156.79(s),154.92(s),148.25(s),136.07(s),125.78(s),106.49(s),63.09(s),38.80( s),19.16(s),18.30(s).MS(ESI):C 12 h 16 N 4 o 4 M=280.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com