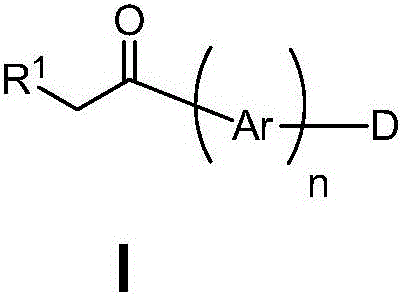
Organic electron luminescent material
A technology of light-emitting materials and organic electronics, applied in the fields of light-emitting materials, organic chemistry, circuits, etc., can solve the problems of high cost and high cost of OLED, and achieve the effect of good charge transport capability, high light-emitting efficiency, and high fluorescence quantum efficiency.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
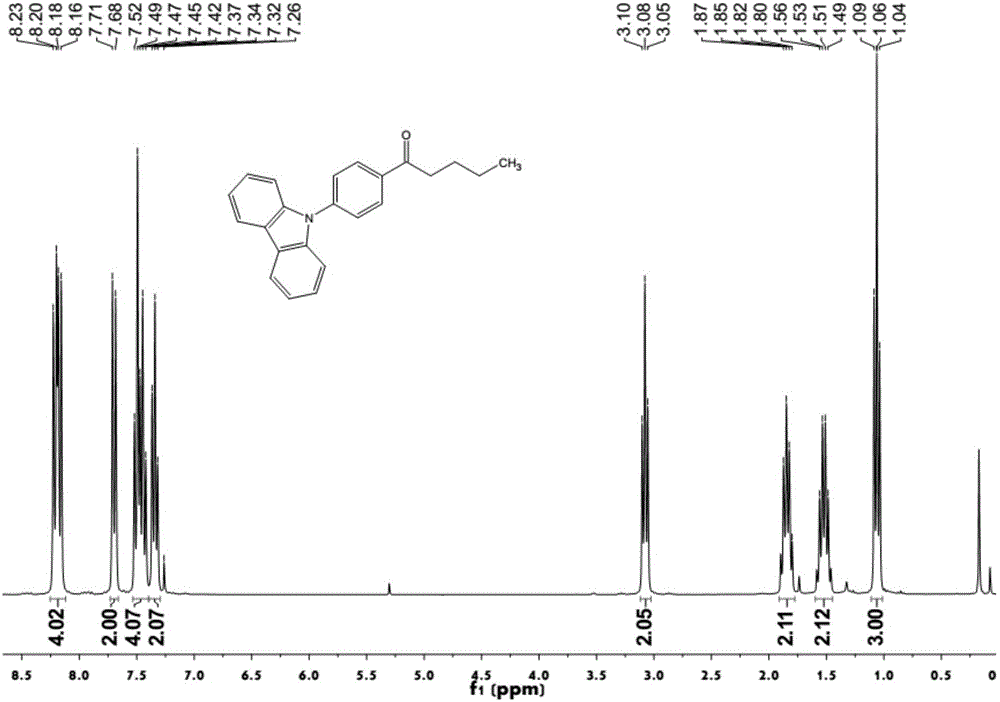
[0044] Embodiment 1: the synthesis of compound 1
[0045]
[0046] Add 2g (0.018mol) of dimethylcarbamoyl chloride and 20ml of tetrahydrofuran solvent to the reaction vessel in turn, deoxygenate the device, pass in nitrogen protection, cool down to the temperature of the reaction solution to -75~-65°C, and slowly add 10ml1 .6Mn-BuLi / THF solution, control the temperature of the reaction solution at -75 ~ -65 ° C, after the dropwise addition, continue to maintain this temperature for 0.5-1h. Finally, 6g of compound 1-1 was added dropwise, and the temperature of the reaction solution was controlled at -75~-65°C. After the dropwise addition, the reaction was continued at this temperature for 0.5-1h, and then the reaction solution was moved to room temperature and reacted for 4-6h. Stop responding. Ethyl acetate / deionized water was added for extraction, and the aqueous layer was extracted with ethyl acetate again. The organic layers were combined, dried over anhydrous magnesium...
Embodiment 2
[0047] Example 2: The following are application examples of the compounds of the present invention.
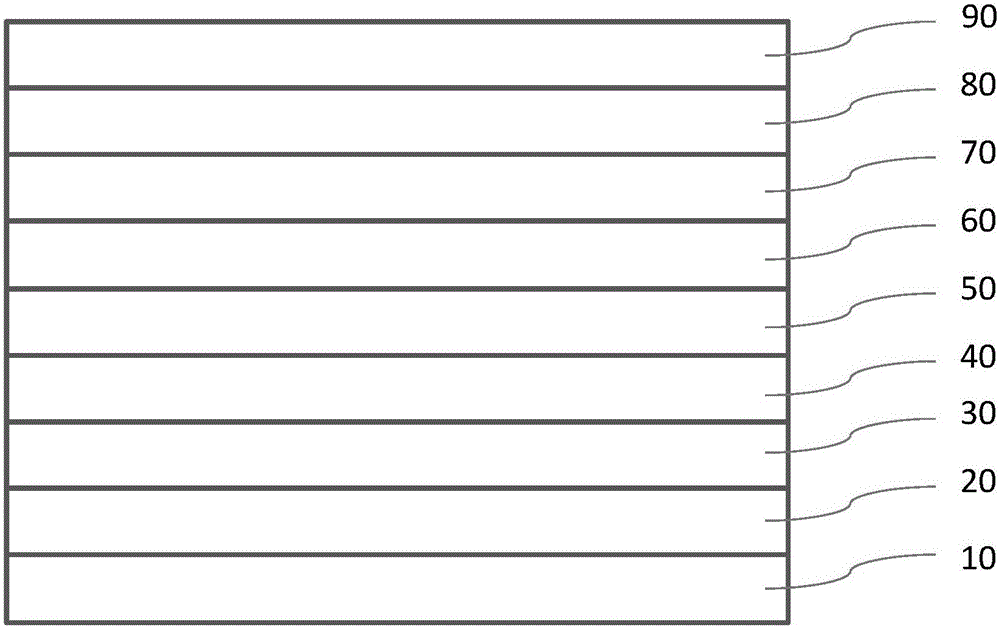
[0048] Device structure such as figure 1 . Device preparation method:
[0049] Firstly, the transparent conductive ITO glass (the glass substrate 10 with the anode 20 ) is cleaned sequentially by detergent solution, deionized water, acetone ultrasonically, isopropanol steam, and then treated with oxygen plasma for 5 minutes.
[0050] Then, 35 nm-thick NPB was vapor-deposited on the ITO as the hole transport layer 30 .
[0051] Then, mCP was evaporated as the electron / exciton blocking layer 40 to a thickness of 5 nm.
[0052] Then, a luminescent layer 50 with a thickness of 20 nm was evaporated, using mCP as the host material, compound 1 of the present invention as the dopant material, and the doping concentration was 3%.
[0053] Then, mCP was vapor-deposited as the exciton blocking layer 60 to a thickness of 10 nm.
[0054] Then, TPBi was vapor-deposited as the electron ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com