Method for catalyzing arenes or heteroarenes to be subjected to trifluoromethylation by semiconductor photocatalysts
A technology for trifluoromethylation and heterocyclic aromatic hydrocarbons, applied in the directions of organic chemistry methods, chemical instruments and methods, preparation of organic compounds, etc. Separation and other problems, to achieve the effect of cheap raw materials, easy separation, and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1 Preparation of semiconductor photocatalyst α-CdS
[0032] Its preparation method comprises the following steps:
[0033] 1) Dissolve 2-5mmol (preferably 2mmol) of cadmium acetate in 100-500mL (preferably 100mL) of deionized water, stir well to dissolve;
[0034] 2) Dissolve 3-7mmol (preferably 3mmol) of sodium sulfide in 100-500mL (preferably 150mL) deionized water, and stir to dissolve;
[0035]3) The sodium sulfide aqueous solution obtained in step 2) is slowly added dropwise to the cadmium acetate aqueous solution obtained in step 1), vigorously stirred for 12-24h (preferably 12h), and then hydrothermally reacted at 200-240°C (preferably 230°C) 24h, centrifuged and washed 5 times with deionized water and absolute ethanol respectively, and dried in a vacuum oven at 60-80°C (preferably 60°C) for 12-24h (preferably 12h). It should be noted that cadmium acetate should be kept in excess during the preparation process.
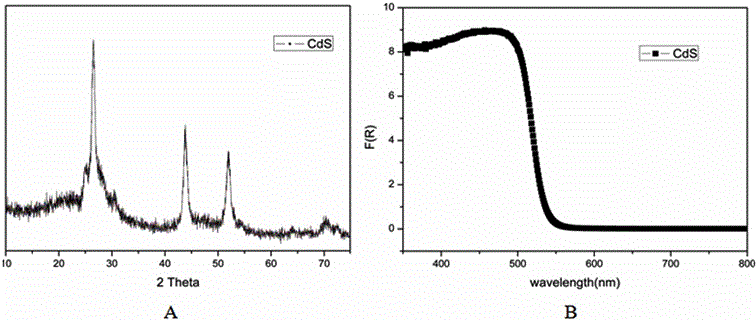
[0036] figure 1 XRD patterns (A) and...
Embodiment 2
[0037] Example 2 Photocatalytic trifluoromethylation reaction with anisole as reaction substrate and CdS as semiconductor photocatalyst
[0038] 1) Weigh out 0.3 mmol of CF 3 SO 2 Na in a 2.5mL centrifuge tube, add 1mL acetonitrile and stir well to dissolve sodium trifluoromethanesulfinate;
[0039] 2) Weigh 10 mg of α-CdS prepared in Example 1 into a 50 mL Schlenk reactor, then add the solution obtained in step 1) into a Schlenk reactor equipped with CdS, and feed oxygen into the reactor for 30 min to make The oxygen in the acetonitrile was saturated, then 0.2 mL of anisole was added, the reactor was sealed after mixing, and the reactor was illuminated under a 300W xenon lamp for 24 hours;
[0040] 3) Centrifuge the reaction solution, take the supernatant for GC-MS and 19 FNMR analysis.
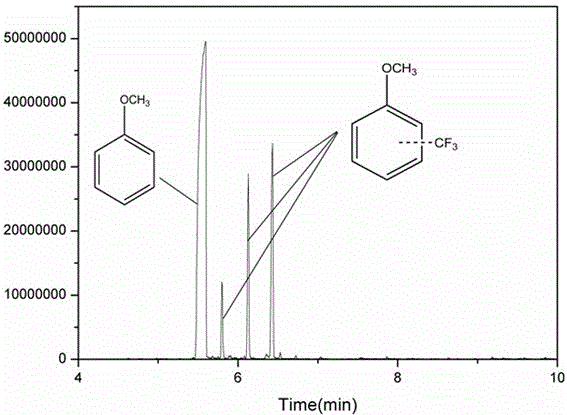
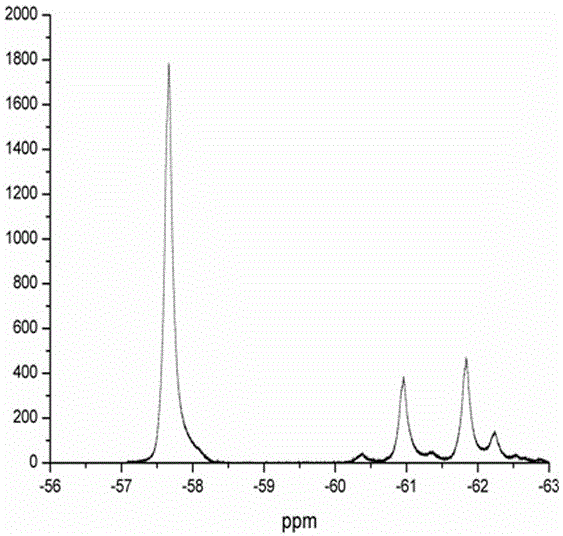
[0041] figure 2 and image 3 are the GC-MS images of the obtained supernatant and 19 FNMR map. As can be seen from the figure, after the reaction is completed, the system composit...
Embodiment 3
[0042] Example 3 Using benzene as a reaction substrate, CdS was subjected to a continuous cycle experiment to test its stability in the reaction system
[0043] 1) Weigh out 0.8 mmol of CF 3 SO 2 Na in a 2.5mL centrifuge tube, add 5mL acetonitrile and stir well to dissolve sodium trifluoromethanesulfinate;
[0044] 2) Weigh 20 mg of α-CdS prepared in Example 1 into a 50 mL Schlenk reactor, then add the solution obtained in step 1) into a Schlenk reactor equipped with CdS, and feed oxygen into the reactor for 30 min to make The oxygen in the acetonitrile was saturated, then 0.5 mL of benzene was added, and the reactor was sealed after mixing, and illuminated under a 300W xenon lamp for 24 hours;
[0045] 3) The reaction solution was centrifuged, and the supernatant was subjected to GC-MS and 19 FNMR analysis;
[0046] 4) Wash the centrifuged solids in step 3) with water and anhydrous ethanol for 5 times each, then vacuum dry at 60°C for 12 hours, and use the dried solids ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com