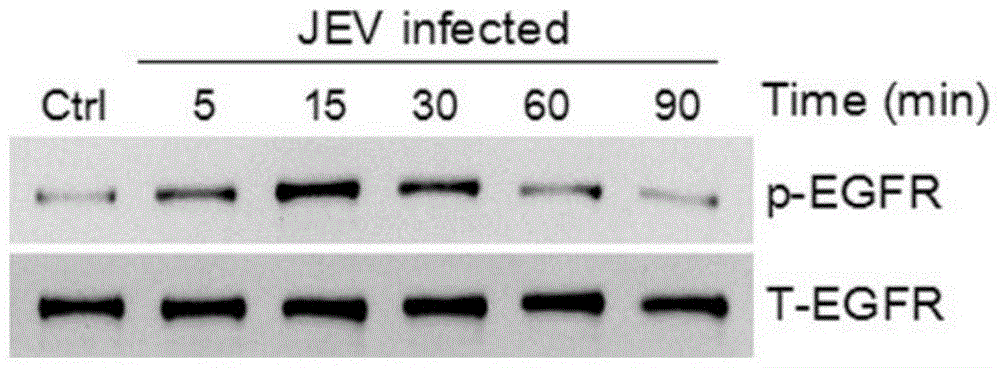
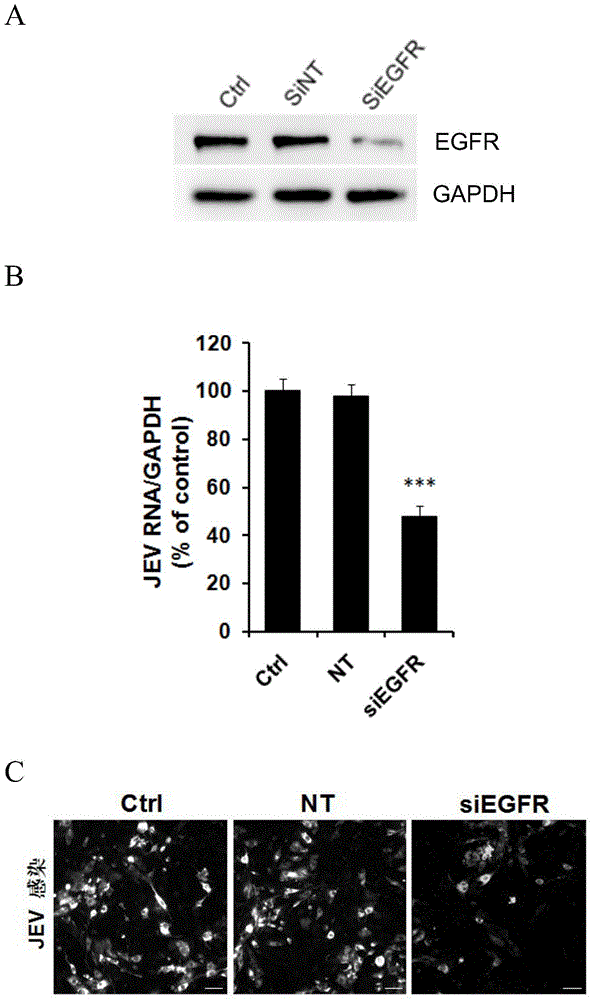
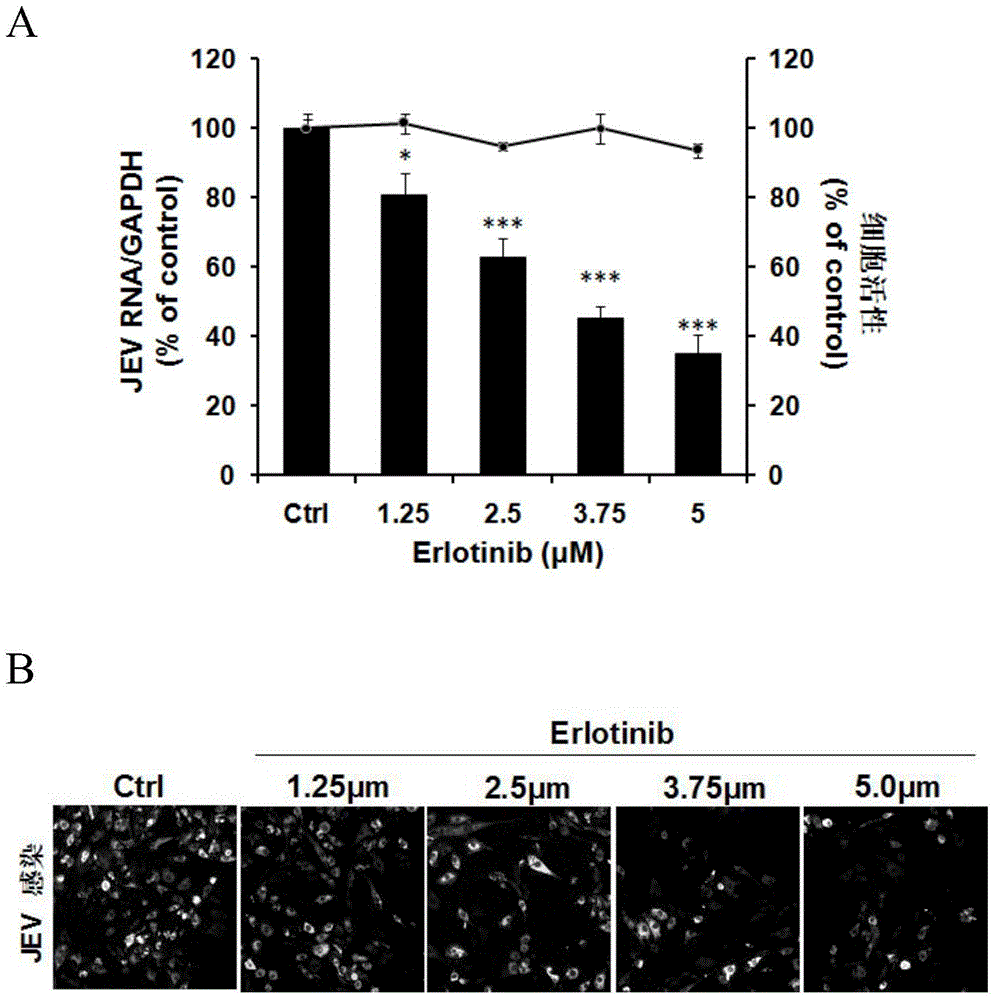
Application of EGFR (Epidermal Growth Factor Receptor) inhibitor in preparation of medicine for treating JE (Japanese Encephalitis)
A Japanese encephalitis and inhibitor technology, applied in the field of medicine, can solve problems such as no literature reports on the effect of EGFR inhibitors, and achieve the effect of good market value and clinical application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] 1. Experimental materials
[0025] Erlotinib is an epidermal growth factor receptor inhibitor.
[0026] Human neuroblastoma line SK-N-SH, purchased from ATCC, accession number: ATCCHTB-11.
[0027] 2. Experimental method
[0028] 1 siRNA interference
[0029] 1.1 RNA transfection
[0030] Refer to Lipofectamine2000 instructions for transfection steps
[0031] 1) 12-16 hours in advance, SK-N-SH cells (purchased from ATCC, deposit number: ATCCHTB-11) were plated and cultured on a 24-well cell culture plate so that the cell density during transfection was 80%-90%.
[0032] 2) Take 2μL of Lipofectamine2000 into 50μL of opti-MEM and mix gently, incubate at room temperature for 5 minutes; take another 5μL of 5μM interfering RNA and mix with 50μL of opti-MEM. After the incubation, add the diluted Lipofectamine2000 transfection reagent to the diluted RNA, and gently pipette to mix. After incubating at room temperature for 20 min, add to SK-N-SH cells and supplement with 400 μL opti-MEM to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com