Trifluoromethyl pyrroloquinoline derivative and synthetic method thereof
A technology of trifluoromethylpyrrole and synthesis method, applied in the direction of organic chemistry, etc., can solve problems such as inability to reflect diversity, and achieve the effect of good yield and high regioselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
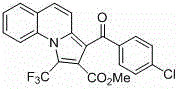
[0027] Add quinoline (65~84.5mg, 0.5~0.65mmol), phenylacetylene (51mg, 0.5mmol), methyl trifluoromethylynoate (114mg, 0.75mmol), cuprous iodide (9.5 mg, 10%mol), dichloromethane (5mL) as solvent, react at room temperature for 24~30 hours; then spin dry and use dimethyl sulfoxide as solvent, add copper bromide (11.2mg, 10%mol), 4-Dimethylaminopyridine (12.2 mg, 20% mol) was heated to 100-120 ° C for 18-24 hours, cooled to room temperature and extracted three times with saturated saline and ethyl acetate, the organic layers were combined and spin-dried, and the column The pure product was isolated by chromatography. Pale yellow solid. Yield 84%.
[0028] Structural formula:
[0029]
[0030] Chinese name: methyl 3-benzoyl-1-(trifluoromethyl)pyrrolo[1,2-a]quinoline-2-carboxylate
[0031] English name:
[0032] methyl3-benzoyl-1-(trifluoromethyl)pyrrolo[1,2-a]quinoline-2-carboxylate
[0033] Molecular weight: 397.09
[0034] Appearance: light yellow solid
[0035] Proto...
Embodiment 2
[0039] Add quinoline (65~84.5mg, 0.5~0.65mmol), p-methylphenylacetylene (58mg, 0.5mmol), methyl trifluoromethylynoate (114mg, 0.75mmol) and iodide in a round bottom flask Copper (9.5mg, 10%mol), dichloromethane (5mL) as solvent, react at room temperature for 24~30 hours; then spin dry and use dimethyl sulfoxide as solvent, add copper bromide (11.2mg, 10% mo), 4-dimethylaminopyridine (12.2 mg, 20% mmol) was heated to 100-120 ° C for 18-24 hours, cooled to room temperature and extracted three times with saturated saline and ethyl acetate, and the organic layers were combined and spun Drying, the pure product was separated by column chromatography. Pale yellow solid. Yield 90%.
[0040] Structural formula:
[0041]
[0042] Chinese name: Methyl 3-(4-methylbenzoyl)-1-(trifluoromethyl)pyrrolo[1,2-a]quinoline-2-carboxylate
[0043] English name:
[0044] methyl3-(4-methylbenzoyl)-1-(trifluoromethyl)pyrrolo[1,2-a]quinoline-2-carboxylate
[0045] Molecular weight: 411.11
[...
Embodiment 3
[0051] Add quinoline (65~84.5mg, 0.5~0.65mmol), p-methoxyphenylacetylene (66mg, 0.5mmol), methyl trifluoromethyl alkynoate (114mg, 0.75mmol), iodide Cuprous (9.5 mg, 10% mol), dichloromethane (5 mL) as solvent, react at room temperature for 24 to 30 hours; then spin dry and use dimethyl sulfoxide as solvent, add copper bromide (11.2 mg, 10 %mol), 4-dimethylaminopyridine (12.2mg, 20%mol) was heated to 100~120°C for 18~24 hours, cooled to room temperature and extracted three times with saturated saline and ethyl acetate, and the organic layers were combined Spin-dry and separate by column chromatography to obtain a pure product. Pale yellow solid. Yield 91%.
[0052] Structural formula:
[0053]
[0054] Chinese name: Methyl 3-(4-methylbenzoyl)-1-(trifluoromethyl)pyrrolo[1,2-a]quinoline-2-carboxylate
[0055] English name:
[0056] Methyl
[0057] 3-(4-methoxybenzoyl)-1-(trifluoromethyl)pyrrolo[1,2-a]quinoline-2-carboxylate
[0058] Molecular weight: 427.10
[0059] A...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com