RGD-peptide-type cationic lipid compound, preparation method thereof, and application of compound to medicine and gene transportation
A technology of cationic lipids and compounds, which is applied in the field of RGD peptide cationic lipids, can solve the problems that cannot meet the requirements of clinical research, monovalent cationic lipids cannot be effective, and the transfection efficiency cannot meet the requirements of treatment, etc., and achieve cell toxicity Low, improve transfection efficiency, and reduce cytotoxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0053] The present invention provides the preparation method of described a kind of RGD peptide cationic lipid compound, and described method comprises the following steps:
[0054] (1) Using a polypeptide solid-phase synthesis instrument, Fmoc-Asp-Wang resin, Fmoc-Gly-OH, and Fmoc-Arg-OH react according to the molar ratio of 1:1:1~1:4:4 to prepare the compound of formula i:
[0055]
[0056] The reaction temperature is 10~40°C, and the reaction solvent is DMF;
[0057] In a preferred embodiment, the reaction temperature is 10~30°C, the reaction solvent is DMF, Fmoc-Asp-Wang resin, Fmoc-Gly-OH, Fmoc-Arg-OH according to the molar ratio of 1:1:1~1: 3:3 response.
[0058] In a more preferred embodiment, the reaction temperature is 15~25°C, the reaction solvent is DMF, Fmoc-Asp-Wang resin, Fmoc-Gly-OH, Fmoc-Arg-OH according to the molar ratio of 1:1:1~1 :2:2 Response.
[0059] (2) Under alkaline conditions, 3-amino-1,2-propanediol and Fmoc-OSu were reacted according to the m...
Embodiment 1
[0091] The preparation of embodiment 1 compound RGDA14
[0092] ⑴ Synthesis of Fmoc-RGD tripeptide
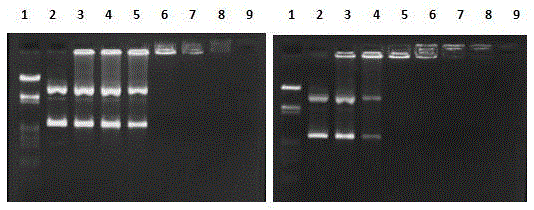
[0093] Using a peptide solid-phase synthesis instrument, put the Fmoc-protected aspartic acid king resin into the reaction kettle, and then prepare the glycine and arginine that need to be connected into a solution, and then prepare the same concentration of HBTU and HOBt and DIEA are added to the container, and the instrument is operated through a computer program to control the reaction. After the reaction was completed, the resin was cut with trifluoroacetic acid, and the pure Fmoc-RGD tripeptide was obtained after purification. The characterization results are as follows: 1 HNMR (400MHz, CDCl 3 ) δ: 8.56 (s, 2H, NH 2 ), 7.28-7.87 (m, 8H, 2×CCHHCHCC), 4.76 (m, 1H, CHCOOH), 4.53 (m, 1H, CHCO), 2.90 (d, 2H, CH 2 COOH).IR, (KBr)υ / cm -1 :3331.15 (υ N-H ), 3054.95 (υ 芳环不饱和C-H ), 2939.33 (υ 饱和C-H ), 1691.52 (υ C=O ), 1614.3, 1537.08 (υ 芳环C=C ), 794.54 (δ 芳环C-H ); ESI-M...
Embodiment 2
[0100] The preparation of embodiment 2 cationic liposomes
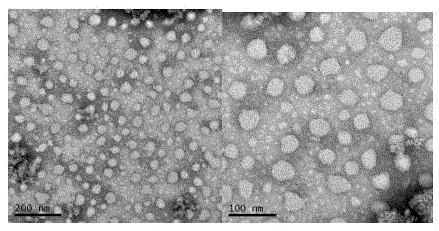
[0101] The RGD peptide-type cationic lipid compound (RGDA14) obtained in the above-mentioned Example 1 was irradiated under ultraviolet light for 1 h, and 1 mg of the cationic lipid was weighed, respectively added to 1 ml of chloroform / methanol (v / v=2:1) fully dissolved, uniformly blown dry with nitrogen to form a thin film, dried in vacuum for 10h, and removed the organic solvent. Add 1ml of sterile ultrapure water, sonicate at a constant temperature of 50°C for 1-3h to make it transparent and clear, and the final concentration is 1.00mM cationic liposome.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com