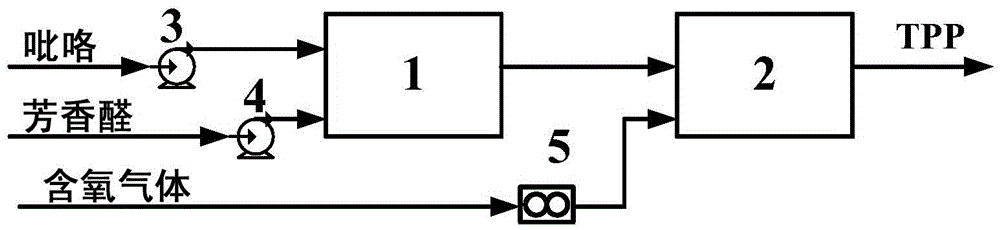
Method for continuously preparing tetraphenyl porphin
A technology of tetraphenylporphine and benzaldehyde, which is applied in the field of continuous preparation of tetraphenylporphine, can solve problems such as difficulty in judging whether the settling tank is full, unrealized continuous material in and out, and large safety hazards of the stirring paddles, etc., to achieve The effect of avoiding the risk of intermittent oxidation operation, avoiding local hot spots, and easy control of reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
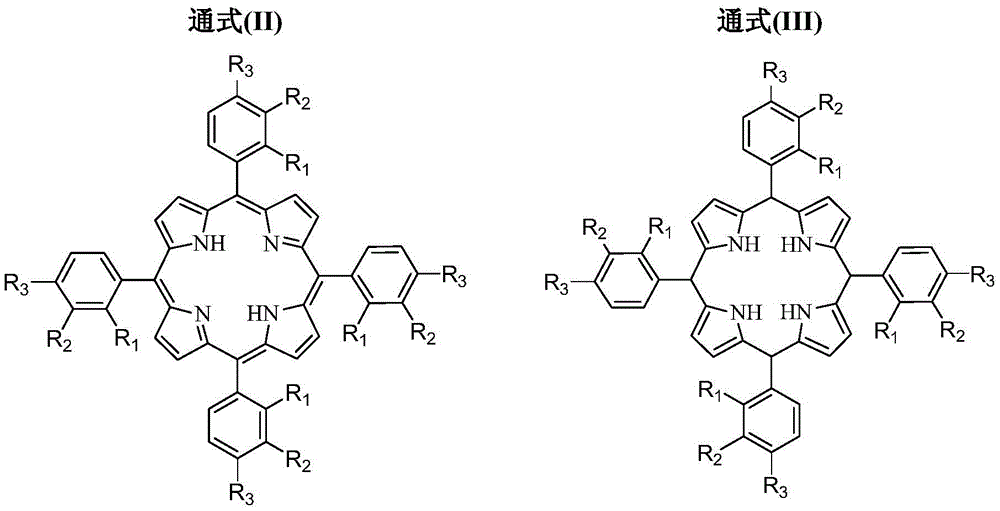
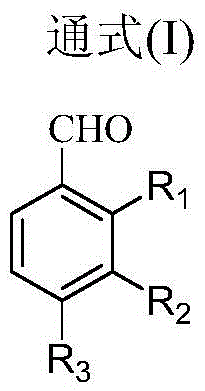
[0056] In the first reactor 1, reaction raw material pyrrole and aromatic aldehyde (R 1 = H, R 2 = H, R 3 =H) Feed in solution form, the solvent is propionic acid, the molar concentration of pyrrole is 0.40mol / L, the molar concentration of aromatic aldehyde is 0.40mol / L, the volume flow rate of pyrrole is 1.05ml / min, the aromatic aldehyde of pyrrole and The molar flow ratio is 1:1. The reaction pressure of the first reactor 1 is 0.83MPa, the reaction temperature is 163.5°C, and the residence time is 244s. The oxygen-containing gas in the second reactor 2 is pure oxygen, and its molar flow ratio to the aromatic aldehyde added in the first reactor 1 is 0.075:1, the reaction pressure of the second reactor 2 is 0.8 MPa, and the reaction temperature is 133.5 ℃, the residence time is 235s. The final synthetic yield of the product TPP based on the reactant pyrrole was 63.9%, and the purity was 98.7%.
Embodiment 2
[0058] In the first reactor 1, reaction raw material pyrrole and aromatic aldehyde (R 1 = H, R 2 = H, R 3 =Cl) feeds in solution form, and solvent is the mixture of propionic acid and toluene, and wherein the volume fraction of propionic acid is 70%, and the molar concentration of pyrrole is 0.10mol / L, and the molar concentration of aromatic aldehyde is 0.20mol / L, and pyrrole The volume flow rate is 2.66ml / min, and the molar flow ratio of pyrrole and aromatic aldehyde is 0.5:1. The reaction pressure of the first reactor 1 is 0.73MPa, the reaction temperature is 179.2°C, and the residence time is 96s. The oxygen-containing gas in the second reactor 2 is pure oxygen, and its molar flow ratio with the aromatic aldehyde added in the first reactor 1 is 1:1, the reaction pressure of the second reactor 2 is 0.70MPa, and the reaction temperature is 149.2 ℃, the residence time is 74s. The final synthetic yield of the product TPP based on the reactant pyrrole was 65.9%, and the puri...
Embodiment 3
[0060] In the first reactor 1, reaction raw material pyrrole and aromatic aldehyde (R 1 = H, R 2 = Cl, R 3 =H) Feed in solution form, solvent is the mixture of propionic acid and benzene, wherein the volume fraction of propionic acid is 80%, the molar concentration of pyrrole is 0.60mol / L, the molar concentration of aromatic aldehyde is 0.60mol / L, pyrrole The volume flow rate is 2.39ml / min, and the molar flow ratio of pyrrole and aromatic aldehyde is 1.0:1. The reaction pressure of the first reactor 1 is 0.90 MPa, the reaction temperature is 176.7° C., and the residence time is 107 s. The oxygen-containing gas in the second reactor 2 is air, and the molar flow ratio between it and the aromatic aldehyde added in the first reactor 1 is 0.9:1, the reaction pressure of the second reactor 2 is 0.87MPa, and the reaction temperature is 176.7°C , the residence time is 25s. The final synthetic yield of the product TPP based on the reactant pyrrole was 66.6%, and the purity was 99.1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com