The preparation method of 4,5-dibromophthalic acid
A technology of bromophthalic acid and phthalic hydrazide, which is applied in the field of preparation of 4,5-dibromophthalic acid, can solve the problems of being unable to meet the batch demand of materials, unfavorable for environmental protection, and outdated methods. Achieve the effect of few steps, easy operation and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
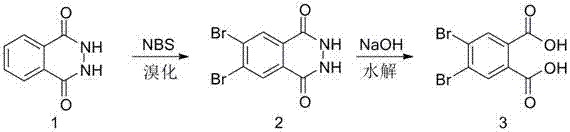
[0018] The preparation method of the 4,5-dibromophthalic acid of the present embodiment, the steps are as follows:
[0019] (1) Synthesis of dibromophthalic hydrazide: Add 16.20 g of phthalic hydrazide into a 250 mL three-necked round-bottomed flask with a reflux condenser, then pour 100 mL of glacial acetic acid into it, stir mechanically, and wait until Add 37.94g of NBS to it after all the solids are dissolved, a little heat will be given off during this process. After the system is stable, turn on the heating and control the temperature at 85°C. As the reaction progresses, the color of the solution gradually deepens, gradually changing from light yellow to yellow. After reacting for 1 h, the reaction solution was cooled, and then poured into ice water. After the ice melted, the precipitated solid was suction filtered to obtain a white solid. After drying, the quality after claiming its drying is 31.8g, and its yield is 99%
[0020] (2) Synthesis of 4,5-dibromophthalic ac...
Embodiment 2
[0023] The preparation method of the 4,5-dibromophthalic acid of the present embodiment, the steps are as follows:
[0024] (1) Synthesis of dibromophthalic hydrazide: Add 16.20 g of phthalic hydrazide into a 250 mL three-necked round-bottomed flask with a reflux condenser, then pour 100 mL of glacial acetic acid into it, stir mechanically, and wait until After all the solids are dissolved, 35.6g of NBS is added thereto, and a little heat will be given off during this process. After the system is stable, turn on the heating and control the temperature at 85°C. As the reaction progresses, the color of the solution gradually deepens, gradually changing from light yellow to yellow. After reacting for 1 h, the reaction solution was cooled, and then poured into ice water. After the ice melted, the precipitated solid was suction filtered to obtain a white solid. After drying, claim that its dried quality is 30 g, and its yield is 94%;
[0025] (2) Synthesis of 4,5-dibromophthalic ...
Embodiment 3
[0027] The preparation method of the 4,5-dibromophthalic acid of the present embodiment, the steps are as follows:
[0028] (1) Synthesis of dibromophthalic hydrazide: Add 16.20 g of phthalic hydrazide into a 250 mL three-necked round-bottomed flask with a reflux condenser, then pour 100 mL of glacial acetic acid into it, stir mechanically, and wait until Add 37.94g of NBS to it after all the solids are dissolved, a little heat will be given off during this process. After the system is stable, turn on the heating and control the temperature at 85°C. As the reaction progresses, the color of the solution gradually deepens, gradually changing from light yellow to yellow. After reacting for 0.5 h, the reaction solution was cooled, and then poured into ice water. After the ice melted, the precipitated solid was suction filtered to obtain a white solid. After drying, the quality after claiming its drying is 28.8g, and its yield is 90%;
[0029] (2) Synthesis of 4,5-dibromophthalic...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com