MOFs method used for deep removing of dibenzothiophene sulfides in automobile diesel oil
A dibenzothiophene and sulfide technology, applied in chemical instruments and methods, copper organic compounds, zinc organic compounds, etc., can solve the problems of Cu-BTC pore blockage and poor mass transfer performance, and achieve enhanced adsorption and mass transfer The effects of rate, pore structure order, and large adsorption capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Cr(NO 3 ) 3 9H 2 O. Terephthalic acid, hydrofluoric acid and water are stirred at a molar ratio of 1:1:1:280 for 30 minutes and then coated on a copper plate with a thickness of 1 mm. The copper plate is placed in a stainless steel adsorption column and heated at 250 ° C Reacted for 12 hours, when the initial sulfur concentration was 350ppm, the saturated sulfur capacity of the modified organic framework p-dibenzothiophene in Case 1 was 150.08mg-DBT / g ads , the saturated sulfur capacity of metal-organic framework p-dibenzothiophene before modification is 96.2mg-DBT / g ads .
Embodiment 2
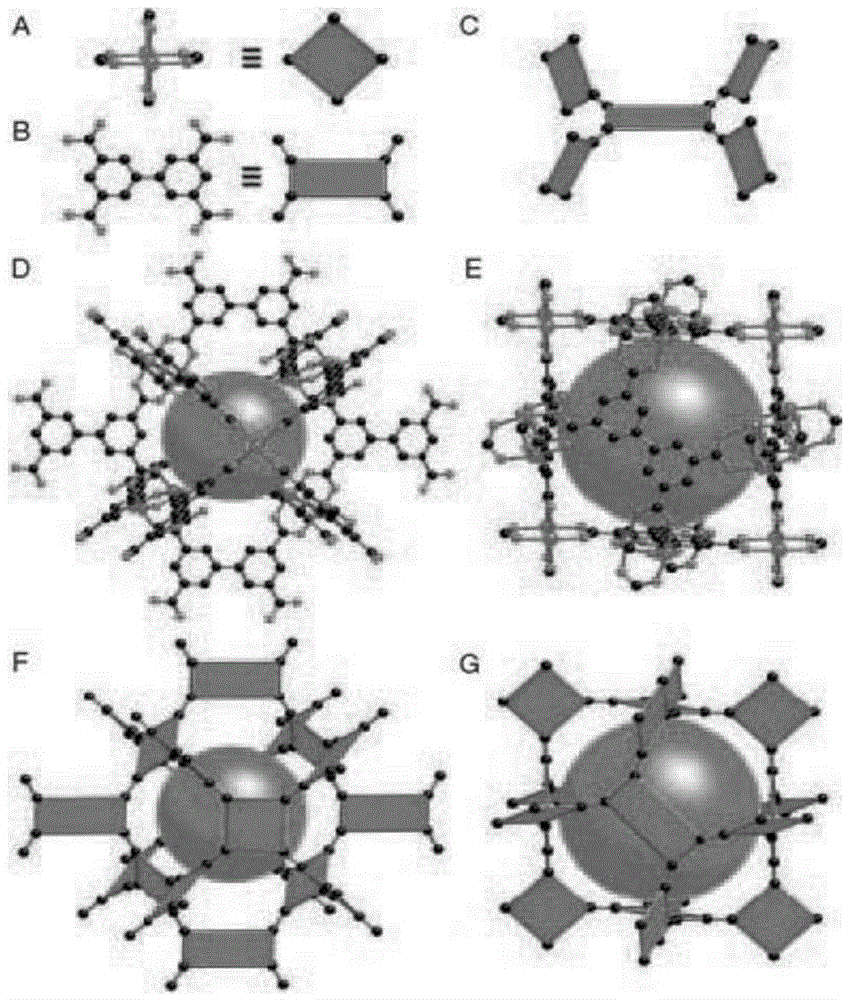
[0025] 0.22mmol of Cu(NO 3 ) 2 2.5H 2 O and 0.076mmol of 3,3,5,5-biphenyltetracarboxylic acid were dissolved in N,N-dimethylformamide / ethanol / water (volume ratio 3:2:2), at 30°C Stir for 45 minutes, and then apply it on an aluminum plate with a thickness of 1.5 mm. Keep the reaction temperature at 50° C. for 48 hours to synthesize a three-dimensional metal-organic framework by hydrothermal synthesis. The metal-organic framework and unit cell structure synthesized in Case 2 are as follows figure 1 shown. The adsorption sulfur capacity of the modified metal-organic framework for 4-methyldibenzothiophene is 8.9 mg / gads, which is 210% of the adsorption sulfur capacity of the unmodified metal-organic framework.
Embodiment 3
[0027] Zn(NO 3 ) 2 ·6H 2 O and 4,4,4-tris(N,N-bis(4-carboxyphenyl)-amino)triphenylamine dissolved in N,N-dimethylacetamide and water, Zn(NO 3 ) 2 ·6H 2 O, 4,4,4-tris(N,N-bis(4-carboxyphenyl)-amino)triphenylamine, N,N-dimethylacetamide and water in a molar ratio of 1:20:10: 10. Mix evenly. After stirring, apply it on an iron plate with a thickness of 0.5 mm. Under nitrogen atmosphere, heat up to 220°C and react for 24 hours to obtain a three-dimensional network topology. The unit cell structure of the synthesized MOFs is as follows: figure 2 shown. In Case 3, the adsorption capacity of 4,6-dimethyldibenzothiophene in diesel reached 20.0mg / g ads , has almost no adsorption capacity for alkanes and aromatics.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com