Compound containing quinoxaline group and organic electroluminescent device thereof
A technology of quinoxaline groups and compounds, applied in the field of organic electroluminescent compounds and organic electroluminescent devices, can solve the problems of low electron mobility, low electron mobility, affecting the life and efficiency of devices, etc. The effect of luminous purity, high luminous efficiency and good thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Synthesis of compound 2
[0045]
[0046] Synthesis of Intermediate 2-1
[0047] In the flask, add 4-bromo-o-phenylenediamine (18.6g, 0.1mol), benzil (21g, 0.1mol) and acetic acid (200ml), heat to 90°C for 5 hours, cool, filter, and use Ethanol was recrystallized to obtain 28 g of the product with a yield of 78%.
[0048] Synthesis of intermediate 2-2
[0049] In a flask, add Intermediate 2-1 (18g, 50mmol), pinacol diboronate (15.2g, 60mmol), potassium acetate (9.8g, 100mmol), dioxane (200ml) and triphenyl Phosphine palladium dichloride (1 g) was heated to reflux for 5 hours under the protection of nitrogen, cooled and concentrated. The crude product was purified by column chromatography to obtain 15.3 g of the product with a yield of 75%.
[0050] Synthesis of Intermediate 2-3
[0051] In a three-necked flask, add 2,4-dichloroquinazoline (6g, 30mmol), biphenylboronic acid (6g, 30mmol), potassium carbonate (8.3g, 60mmol), tetrakistriphenylphosphine palladium (0.3...
Embodiment 2
[0055] Synthesis of compound 7
[0056]
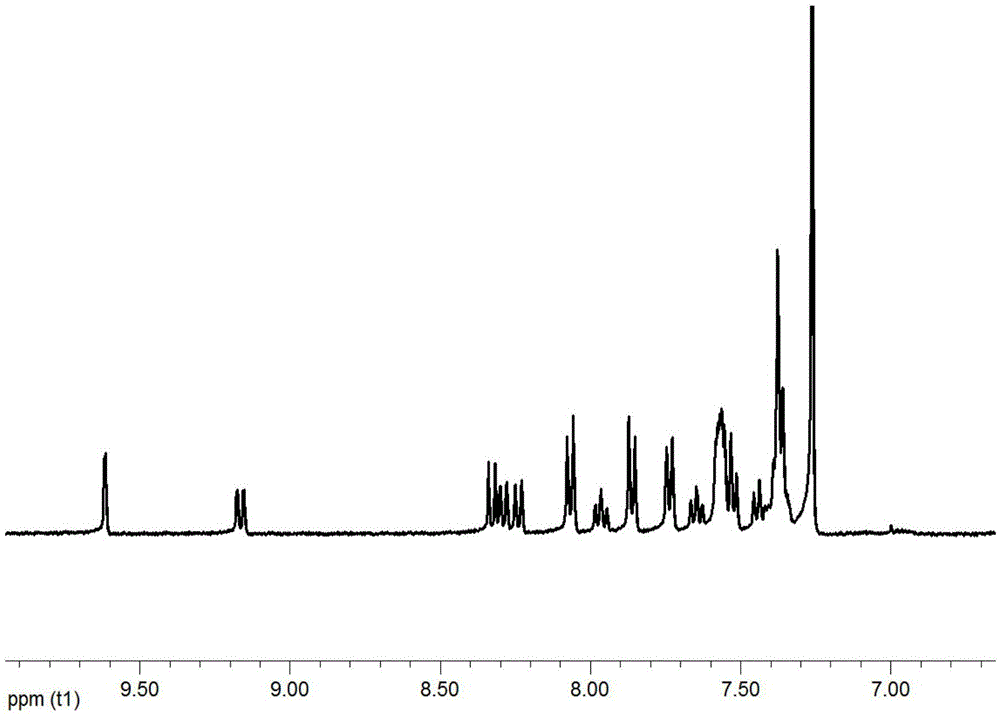
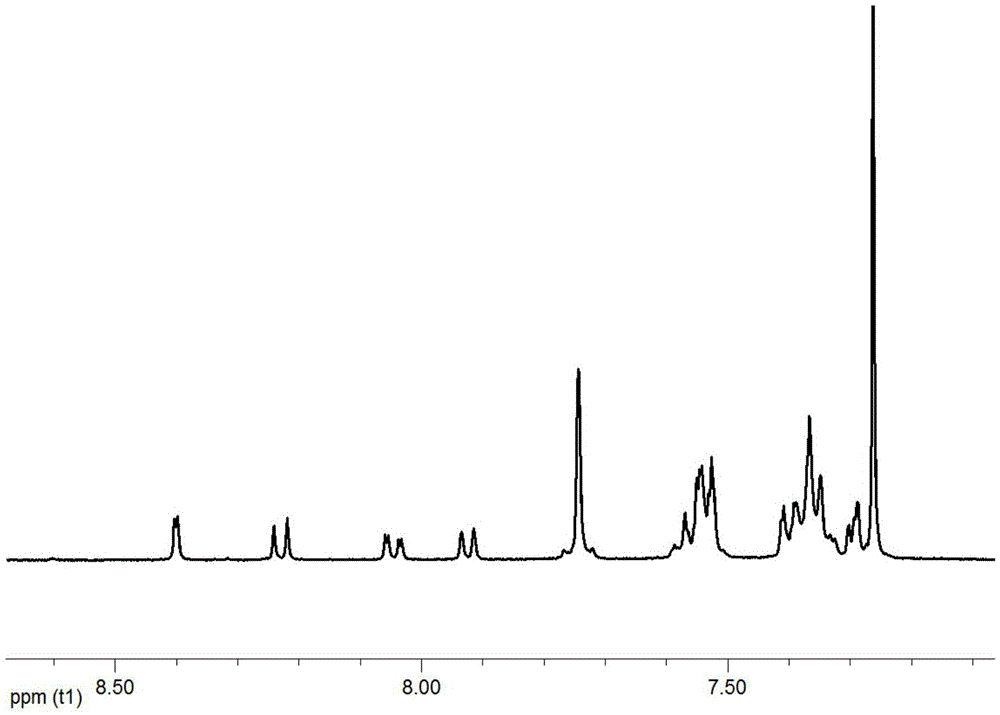
[0057] In a three-necked flask, add intermediate 2-2 (2g, 4.9mmol), 2-(4-bromophenyl)-1-phenyl-1H-benzimidazole (1.7g, 4.9mmol), potassium carbonate (1.1 g, 10mmol), tetrakistriphenylphosphine palladium (0.1g), tetrahydrofuran (50ml) and water (20ml), heated for 5 hours under nitrogen protection, cooled, extracted with dichloromethane, dried, filtered, and the crude product was passed through the column Analyze and purify to obtain product 2.3g, productive rate 85%, its proton magnetic spectrum figure is as follows figure 2 shown.
Embodiment 3
[0059] Synthesis of compound 17
[0060]
[0061] In a three-necked flask, add intermediate 2-2 (2g, 4.9mmol), 2-(4-bromophenyl)-4,6-diphenyl-1,3,5-triazine (1.9g, 4.9mmol ), potassium carbonate (1.1g, 10mmol), tetrakistriphenylphosphine palladium (0.1g), tetrahydrofuran (50ml) and water (20ml), heated for 5 hours under nitrogen protection, cooled, extracted with dichloromethane, dried, filtered , the crude product was purified by column chromatography to obtain 1.9 g of the product, with a yield of 67%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com