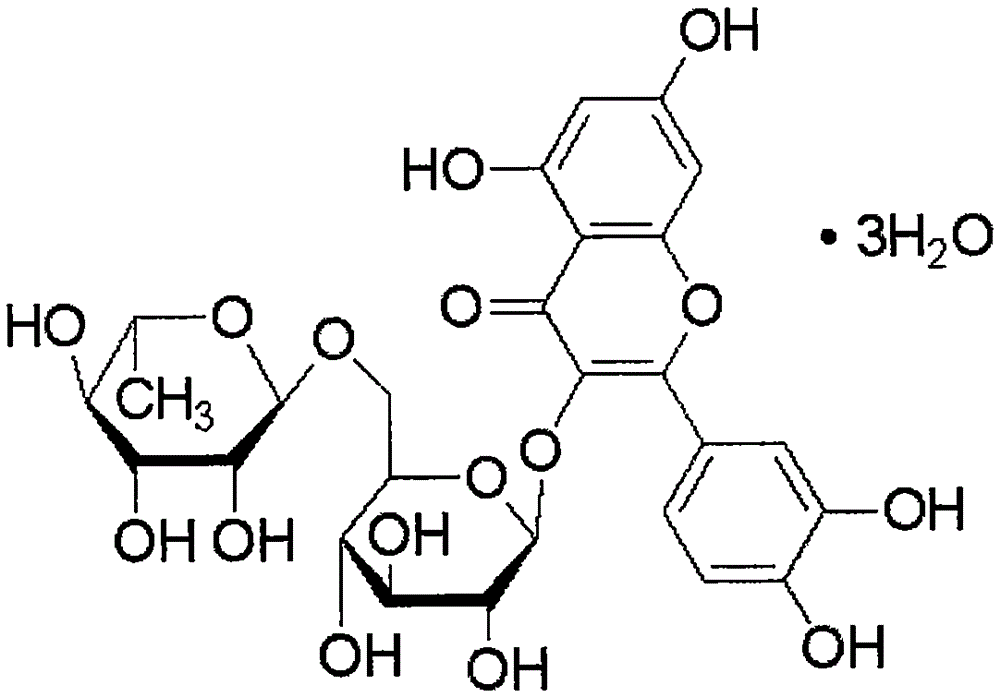
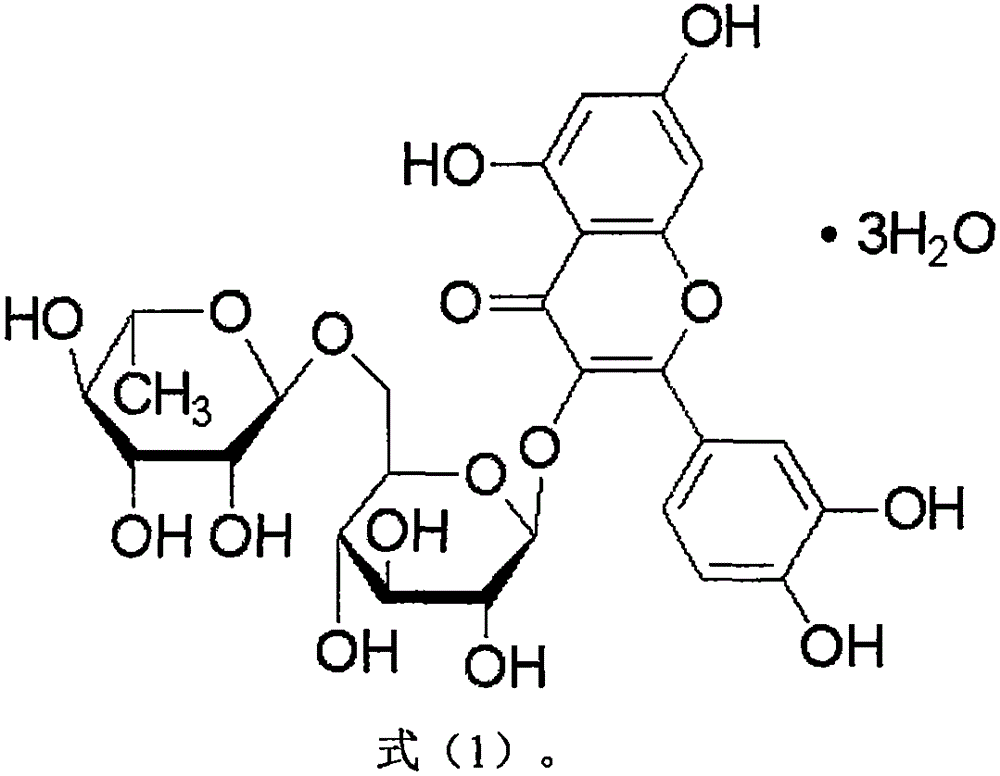
Application of rutoside in preparation of osteosarcoma treating drug
A treatment drug and osteosarcoma technology, applied in the preparation of osteosarcoma treatment drugs, the field of rutin, can solve problems such as orthopedic diseases or tumor treatment effects that have not yet been found
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1 (in vivo inhibition experiment of rutin to osteosarcoma cells)
[0028] 1 test material
[0029] 1.1 Experimental animals
[0030] SPF grade experimental mice were purchased from the market.
[0031] 1.2 Experimental osteosarcoma cells
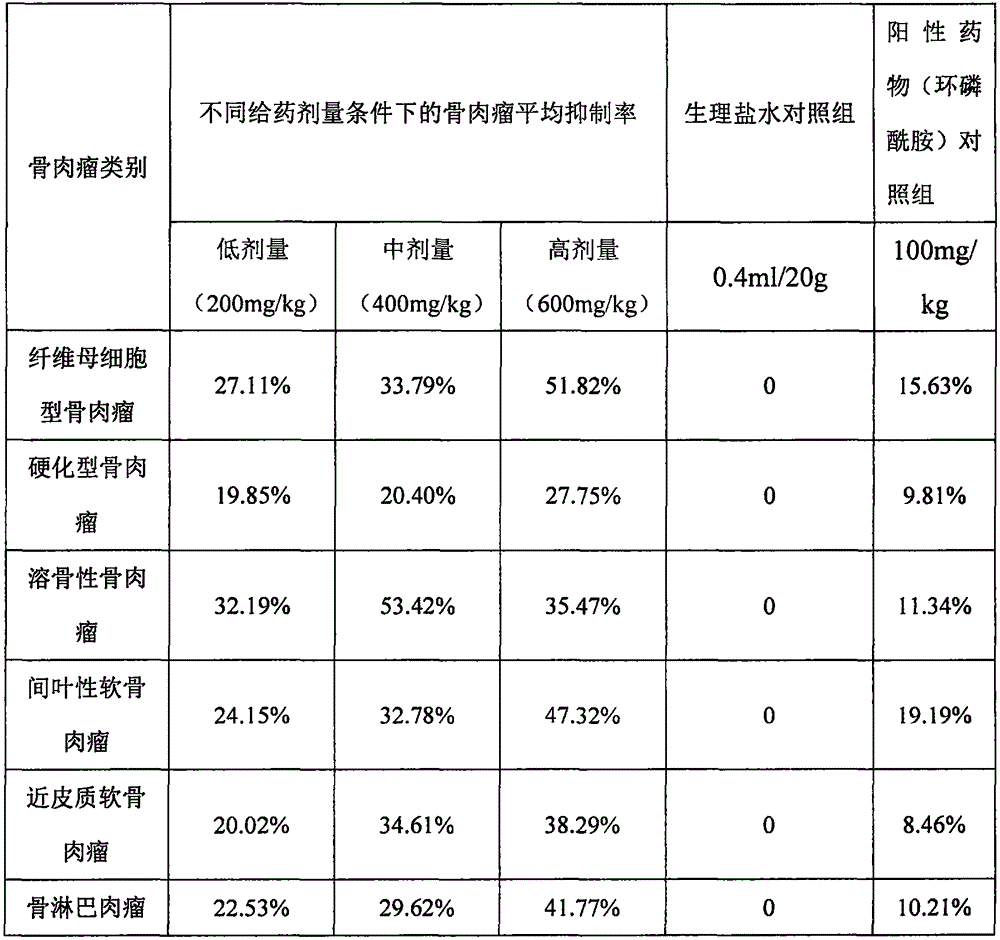
[0032] The osteosarcoma cells used in the experiment include: fibroblastic osteosarcoma; sclerosing osteosarcoma; osteolytic osteosarcoma; mesenchymal chondrosarcoma; juxtacortical chondrosarcoma;
[0033] 1.3 Test samples
[0034] Pure rutin, sealed and stored at 4°C. When in use, prepare a solution of the required concentration with distilled water at about 35-40°C for intragastric administration to animals, and store in a refrigerator at 4°C. The drug can be used within 7 days after preparation.
[0035] 1.4 Drugs and reagents
[0036] Positive control drug: cyclophosphamide for injection, powder injection in ampoule, 0.2g / bottle. When in use, it is prepared with physiological saline to make a medicinal solution of...
Embodiment 2
[0051]Embodiment 2 (in vitro inhibition experiment of rutin to osteosarcoma cells)
[0052] 1 Experimental materials
[0053] 1.1 Samples to be tested
[0054] Pure rutin, packed in a sealed plastic box, stored in a refrigerator at 4°C. When used, it is prepared with 1640 culture medium containing 10% calf serum and diluted in multiples for use in cell experiments, and it is freshly prepared when used. The period of use after the preparation of the medicine is 3 to 4 days.
[0055] 1.2 Osteosarcoma cell lines
[0056] Osteosarcoma cells include: fibroblastic osteosarcoma; sclerosing osteosarcoma; osteolytic osteosarcoma; mesenchymal chondrosarcoma; juxtacortical chondrosarcoma; In the 1640 culture medium of serum, passaging once every 2 days, and the cells in the logarithmic growth phase were taken for experiments.
[0057] 1.3 Instruments and reagents
[0058] Reagent:
[0059] Improved RPMI1640 culture medium; mycoplasma-free newborn bovine serum; MTT solution; dimeth...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com