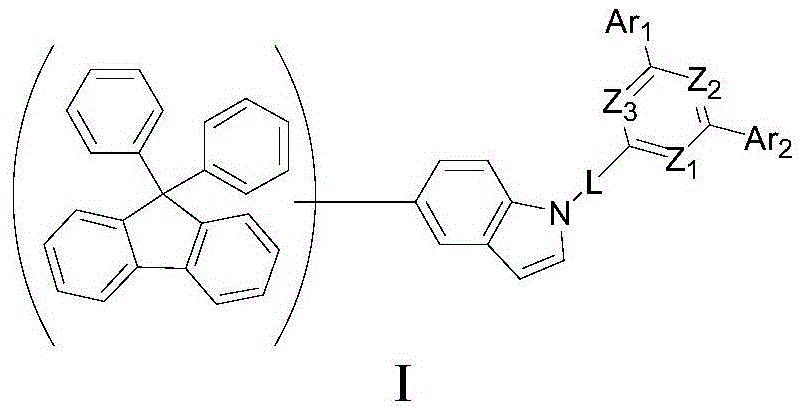
Phosphorescence main body compound containing indole groups and organic electroluminescent device of phosphorescence main body compound
A technology of phosphorescent host and indole group, applied in the field of phosphorescent host compounds and organic electroluminescent devices, can solve the problems of unbalanced charge in the light-emitting layer, reduced device efficiency, difficult electron flow, etc., and achieves high light-emitting purity, good The effect of thermal stability and high luminous efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
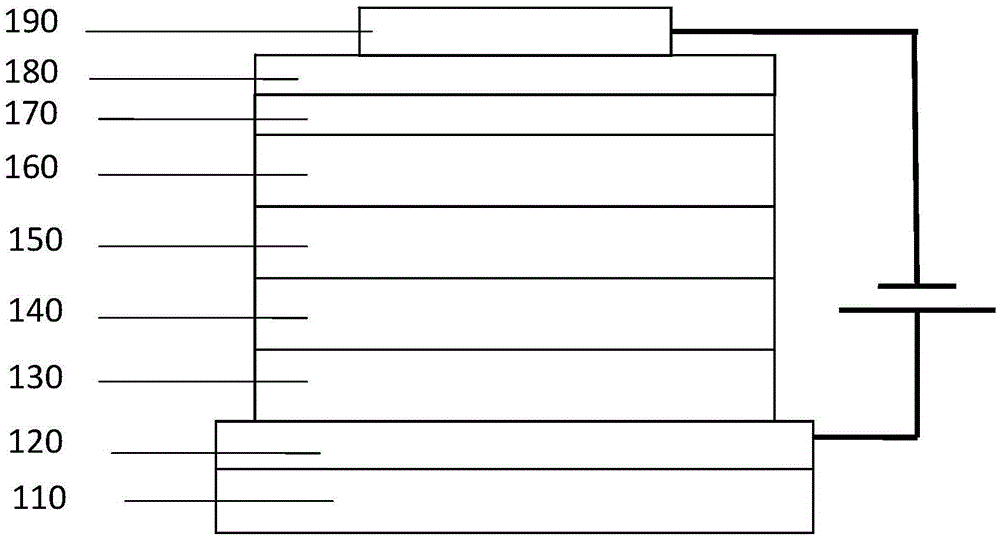
Image
Examples
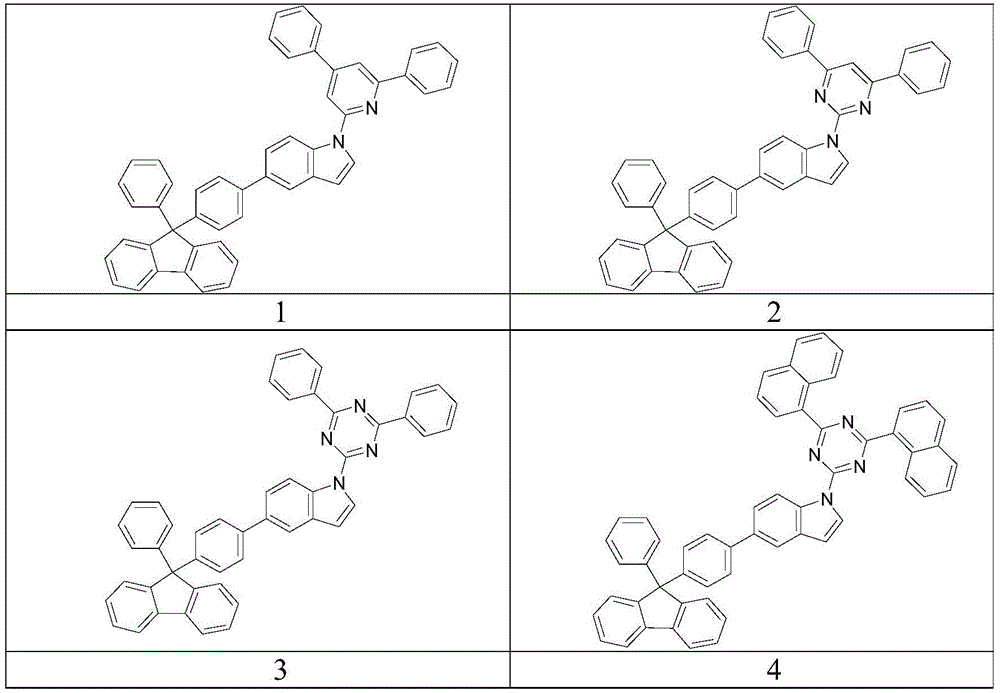
Embodiment 1
[0050] Synthesis of compound 2
[0051]
[0052] Synthesis of Intermediate 2-1
[0053] In a 500mL single-neck flask, add 5-bromoindole (20.00g, 0.1mol), pinacol diborate (51.80g, 0.2mmol), potassium acetate (22.80g, 0.2mol), [1,1 '-Bis(diphenylphosphino)ferrocene]palladium dichloride (1.50g), 350mL dioxane, reflux for 8h under nitrogen protection. After cooling, the dioxane was removed by rotary evaporation, washed with 200 mL of distilled water, and extracted with dichloromethane three times. The product was recrystallized from methanol-dichloromethane to obtain 21.8 g of the product, and the yield was 90%.
[0054] Synthesis of Intermediate 2-2
[0055] In a 50mL single-necked flask, add Intermediate 2-1 (2.4g, 10mmol), 9-phenyl-9-(4-bromophenyl) fluorene (4.4g, 11mmol), potassium carbonate (2.7g, 20mmol) ), palladium tetrakistriphenylphosphorus (50mg), dioxane (20.0mL) and water (4.0mL), reflux for 5h under nitrogen protection. After cooling, the dioxane was removed by rotary ...
Embodiment 2
[0059] Synthesis of compound 3
[0060]
[0061] The synthesis method is the same as that of compound 2, except that 2-chloro-4,6-diphenyl-s-triazine is used instead of 2-chloro-4,6-diphenylpyrimidine, the yield is 85%.
Embodiment 3
[0063] Synthesis of compound 18
[0064]
[0065] In a three-necked flask, add Intermediate 2-2 (2g, 4.6mmol), 2-(3-bromophenyl)-4,6-diphenyl-1,3,5-triazine (1.8g, 4.6mmol) ), potassium carbonate (1.2g, 9.2mmol), cuprous iodide (0.3g), o-phenanthroline (0.3g) and nitrobenzene (30ml), heated to reflux for 12 hours, cooled, and removed the solvent under reduced pressure. The product was purified by column chromatography to obtain a yield of 2.3 g and a yield of 68%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com