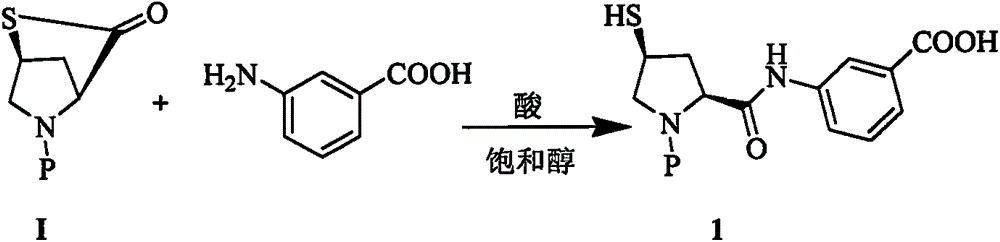
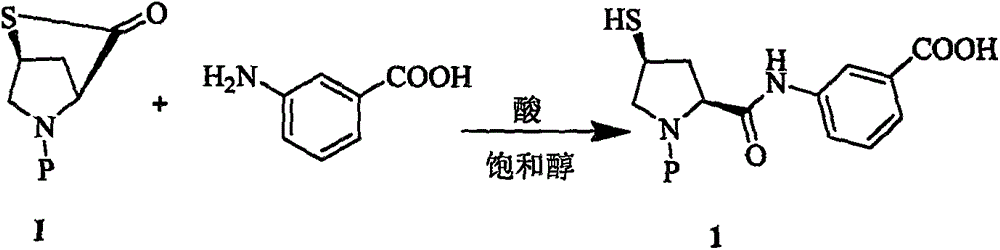
Improved manufacturing method of ertapenem side chain
A technology of ertapenem side chain and raw material is applied in the field of preparation of carbapenem antibiotic side chain, and can solve the problems of excessive waste water, high product cost, environmental pollution and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] Example 1: Preparation of Compound 1
[0015] At 60°C, to 200g of methanol solution containing meta-aminobenzoic acid (9.45g, 68.9mmol), add 1g of tributylphosphine and 400ml of 4MHCl / methanol solution, and then slowly add raw material I (19.8g, 64.28mmol) Then, the reaction mixture was stirred for 3h at the same temperature. The slurry reaction solution was cooled and filtered to obtain white crystals. The white crystals were washed with methanol and dried in vacuum to obtain 127.8 g of compound. The molar yield was 97% and the HPLC purity was 99.2. %
[0016] MS: 446.1[M+1], 444.3[M-1]
[0017] 1 H-NMR (400MHz, DMSO-d6): δ 1.85 (m, 1H), 2.70 (m, 1H), 3.27 (m, 2H), 3.97 (m, 1H), 4.35 (m, 1H), 5.13 ( m, 1H), 7.82 (m, 8H), 10.28 (d, J = 9.0 Hz, 1H).
Embodiment 2
[0018] Example 2: Preparation of Compound 2
[0019] At 70℃, to 120g of ethanol solution containing meta-aminobenzoic acid (5.59g, 40.8mmol), add 0.5g of tributylphosphine and 200ml of 4MHCl / 7 alcohol solution, and then slowly add raw material I (12.32g40mmol) 150g of ethanol solution, then stirred and reacted at the same temperature for 2.5h, the slurry reaction solution was cooled and filtered to obtain white crystals, which were washed with ethanol and dried in vacuum to obtain 116.8g of compound, molar yield 94.8%, HPLC purity 99.4 %
Embodiment 3
[0020] Example 3: Preparation of Compound 2
[0021] At 80°C, to 150 g of a propanol solution containing meta-aminobenzoic acid (11.18 g, 81.6 mmol), 1.2 g of tributyl phosphine and 140 g of acetic acid were added, and then slowly added the propylene of raw material I (24.64 g, 80 mmol). 400g of alcohol solution, then stirred and reacted at the same temperature for 2h. The slurry reaction solution was cooled and filtered to obtain white crystals. The white crystals were washed with propanol and dried in vacuum to obtain 134.25g of compound. The molar yield was 96% and the HPLC purity was 99.5. %.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com