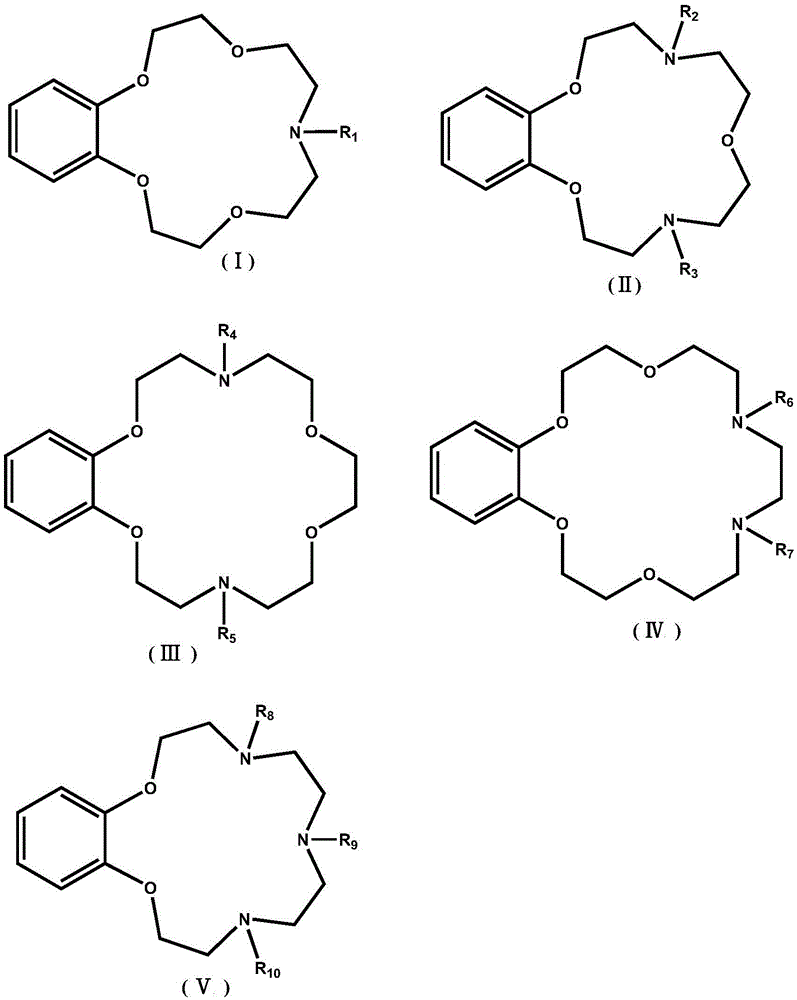
Application of benzo-azacrown ether compounds to separation of lithium isotopes
A technology of lithium isotope and heterocrown ether, which is applied in the field of lithium isotope separation, can solve the problems of complex synthesis route and high cost of extraction agent, and achieve the effects of fast isotope exchange rate, simple operation and simple process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
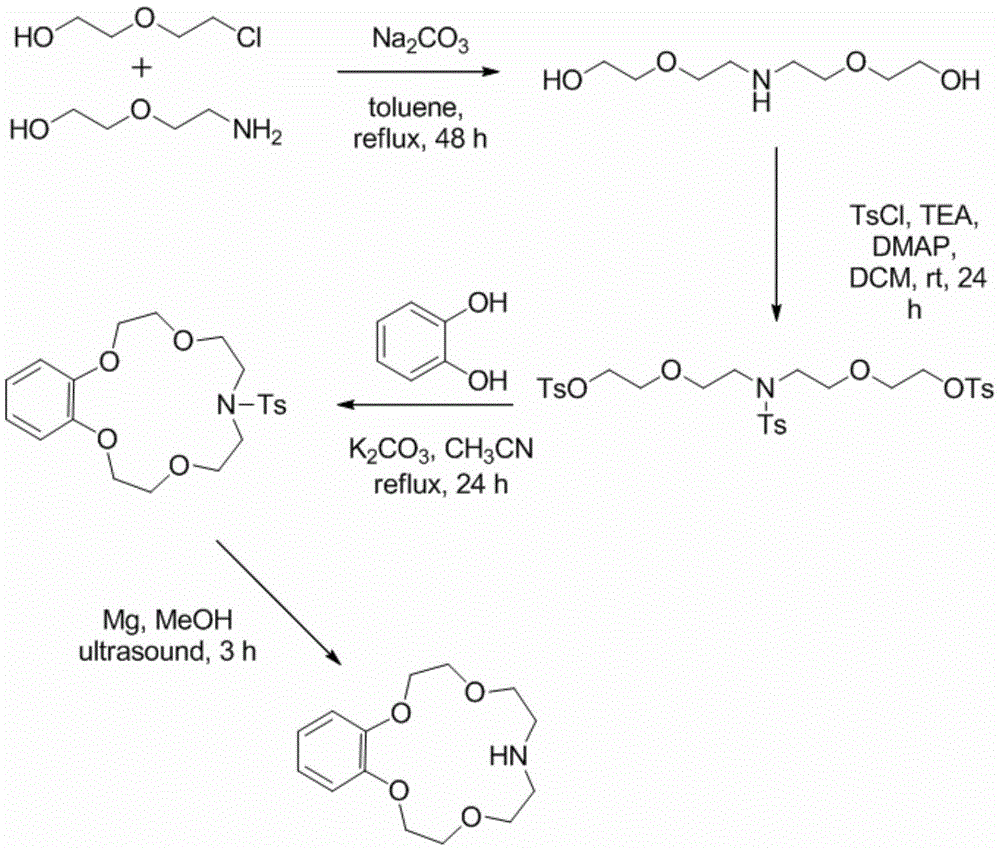
[0024] Synthesis and Lithium Isotope Separation Properties of Benzomonaazepine-15-Crown-5. The reaction equation is as follows:
[0025]
[0026] Concrete reaction process is as follows:
[0027] Dissolve 5.0g of 2-chloroethoxyethanol, 4.22g of diglycolamine, and 17.0g of sodium carbonate in 100mL of toluene, heat to reflux, and react for 48h. When the temperature drops to room temperature, filter out sodium carbonate, distill off toluene under reduced pressure, add 200mL water to the residue and extract with dichloromethane, dry the extract phase with anhydrous sodium sulfate, add 16.7mL, 22.9g 4-methylbenzenesulfonyl chloride and 100mg4 -Dimethylaminopyridine, after reacting at room temperature for 24 hours, add an appropriate amount of dilute hydrochloric acid, separate the organic phase, extract the aqueous phase with dichloromethane, wash the organic phase twice with saturated NaCl aqueous solution, and dry over anhydrous sodium sulfate. The solvent was evaporated un...
Embodiment 2
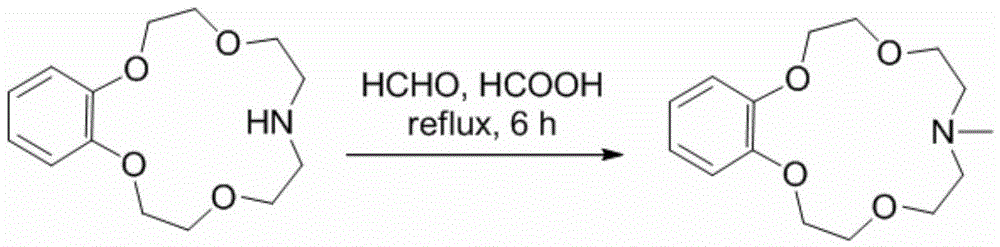
[0030] Synthesis and lithium isotope separation properties of N-methylbenzomonoazepine-15-crown-5. The reaction equation is as follows:
[0031]
[0032] Concrete reaction process is as follows:
[0033] Dissolve 2 g of benzodiazepine-15-crown-5 in 20 mL of formic acid and 20 mL of formaldehyde solution, heat to reflux, and react for 24 hours. When the temperature drops to room temperature, add an appropriate amount of saturated aqueous sodium bicarbonate solution to make the solution alkaline. Then it was extracted with dichloromethane, the extract phase was washed twice with saturated NaCl aqueous solution, and dried over anhydrous sodium sulfate. The solvent was distilled off under reduced pressure to obtain the crude product, which was purified by silica gel column chromatography to obtain 51.7 g of N-methylbenzodiazepine-15-crown-51.7 g, 1 H-NMR (400MHz, CDCl 3 )δ6.94-6.83(m,4H),4.15-4.12(m,4H),3.90-3.87(m,4H),3.77(t,4H),2.74(t,4H),2.35(s,3H) ppm.
[0034] Dissolv...
Embodiment 3
[0036] Synthesis of N-methoxyethylbenzomonoazepine-15-crown-5 and properties of lithium isotope separation. The reaction equation is as follows:
[0037]
[0038] Concrete reaction process is as follows:
[0039] Dissolve 2g of benzomonaazepine-15-crown-5 in 100mL of acetonitrile, add 1.04g of 2-bromoethyl methyl ether, 4.9g of anhydrous cesium carbonate, reflux for 24h, and filter out the carbonic acid after the temperature drops to room temperature cesium, the solvent was evaporated under reduced pressure, and the crude product was purified by silica gel column chromatography to obtain 1.75g of N-methoxyethylbenzodiazepine-15-crown-5, 1 H-NMR (400MHz, CDCl 3 )δ6.94-6.83(m,4H),4.14-4.10(m,4H),3.90-3.84(m,4H),3.77(t,4H),3.47(t,2H),3.33(s,3H) , 2.87(t,4H), 2.78(t,2H)ppm.
[0040]Dissolve N-methoxyethylbenzomonaazepine-15-crown-5 in chloroform to prepare 0.15mol / L chloroform solution as the organic phase, and pass liquid-liquid reaction with 2mol / L lithium trifluoroacet...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com