Vinyl norbornene production method
A technique for producing vinyl norbornene and a production method, which is applied in the field of Diels-Alder reaction of 3-butadiene, can solve the problems of low conversion rate, poor selectivity of target products, harsh reaction conditions, etc., and achieves improved yield, multiple The effect of avoiding the formation of polymers and polymers and improving selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
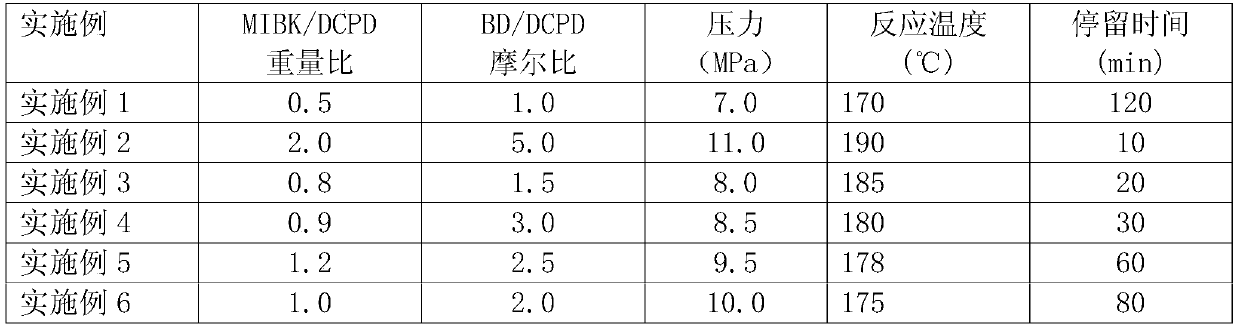
Embodiment 1~6
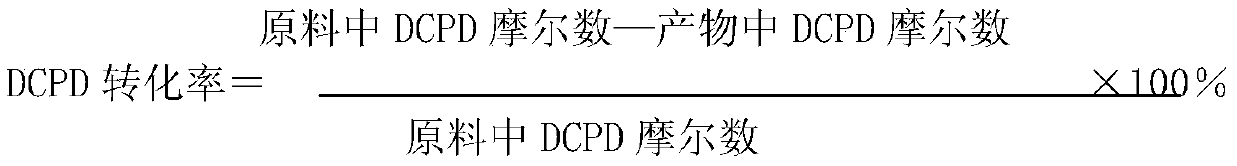
[0031]The reaction was carried out in two tank reactors connected in series with a volume capacity of 2 liters and 1 liter respectively. The reactor was equipped with a stirring device, a cooling coil, and an electric heating system was installed outside. Pump the prepared dicyclopentadiene (DCPD), 1,3-butadiene (BD), solvent methyl isobutyl ketone (MIBK) and p-tert-butylcatechol (TBC) from the first tank The bottom of the reactor is continuously fed into the first tank reactor, the stirring is started and the temperature is raised to the required temperature, and then nitrogen is used to adjust the pressure of the reactor to the set pressure through the constant pressure valve. The reaction liquid in the first tank reactor Continuously enter the second tank reactor through the outlet on the upper side of the reactor through the bottom of the second tank reactor, continue to react under the set conditions, and the reaction product is discharged through the outlet on the upper s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com