A kind of method that catalytic oxidation alicyclic alcohol compound prepares alicyclic ketone
A technology for compounds and alicyclic alcohols is applied in the field of catalytic reaction systems for selectively oxidizing alicyclic alcohol compounds to prepare corresponding alicyclic ketones. Less by-products, mild reaction conditions and less environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
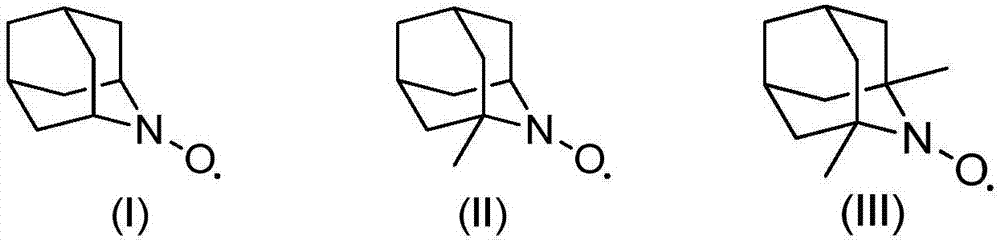
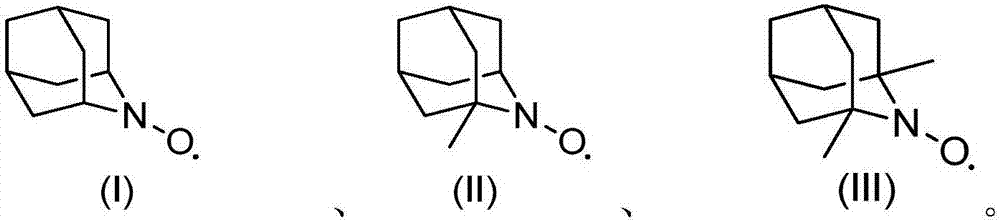
[0013] 1.0g cyclohexanol, 2mol% (relative to the substrate cyclohexanol) azaadamantane type nitroxide free radical (I), 2mol% (relative to the substrate cyclohexanol) vanadyl sulfate, 5mL acetonitrile was added to The reactor was filled with oxygen at a pressure of 0.3 MPa, operated at 80°C for 3 hours, and then cooled to room temperature. Sampling was analyzed by gas chromatography, and the conversion rate of cyclohexanol was 99.5%, and the selectivity of cyclohexanone was 99.9%.
[0014] If use 2,2,6,6-tetramethylpiperidine nitroxide free radical to replace azaadamantane type nitroxide free radical (I), under the same reaction conditions of above-mentioned embodiment 1 (catalyst substrate ratio, reaction temperature , reaction time, oxygen partial pressure, solvent and consumption etc.), the conversion rate of cyclohexanol is only 67%. If the reaction time is further extended to 15 hours, the conversion of cyclohexanol reaches 91%. It can be seen that its oxidation efficie...
Embodiment 2
[0016] With 10g cyclohexanol, 1mol% (relative to the substrate cyclohexanol) azaadamantane type nitroxide radical (I), 1mol% (relative to the substrate cyclohexanol) vanadyl sulfate, 100mL acetonitrile was added to the reaction The kettle was filled with oxygen at a pressure of 0.5MPa, operated at 100°C for 10h and then cooled to room temperature. Sampling was analyzed by gas chromatography, and the conversion rate of cyclohexanol was 99.1%, and the selectivity of cyclohexanone was 99.9%.
Embodiment 3
[0018] With 1.76g 2-phenyl cyclohexanol, 0.05mol% (relative to the substrate 2-phenylcyclohexanol) azaadamantane type nitroxide free radical (II), 0.5mol% (relative to the substrate 2- Phenylcyclohexanol) vanadyl trichloride, 5mL of acetonitrile were added to the reaction kettle, filled with air pressure of 0.5MPa, operated at 80°C for 20h and then cooled to room temperature. Samples were analyzed by gas chromatography, and the conversion rate of 2-phenylcyclohexanol was 99.7%, and the selectivity of 2-phenylcyclohexanone was 99.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com