Medicine composition for improving piglet production performance and preparation method and application of medicine composition
A technology of production performance and composition, applied in the application, animal feed, additional food elements, etc., can solve the problem of small improvement, and achieve the effect of improving production performance and good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Preparation and efficacy of embodiment 1 pharmaceutical composition of the present invention
[0033] 1. Preparation method
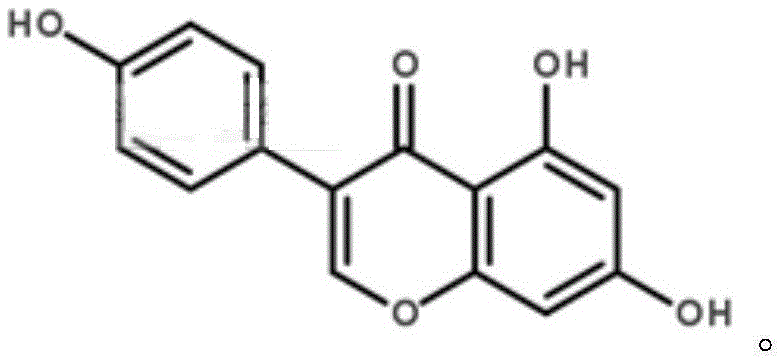
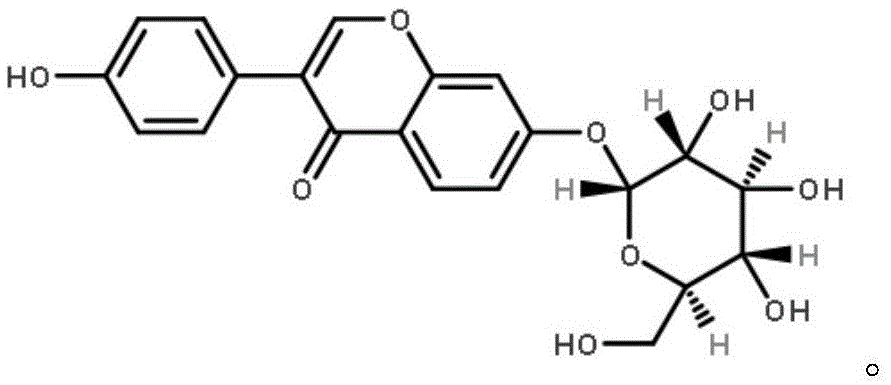
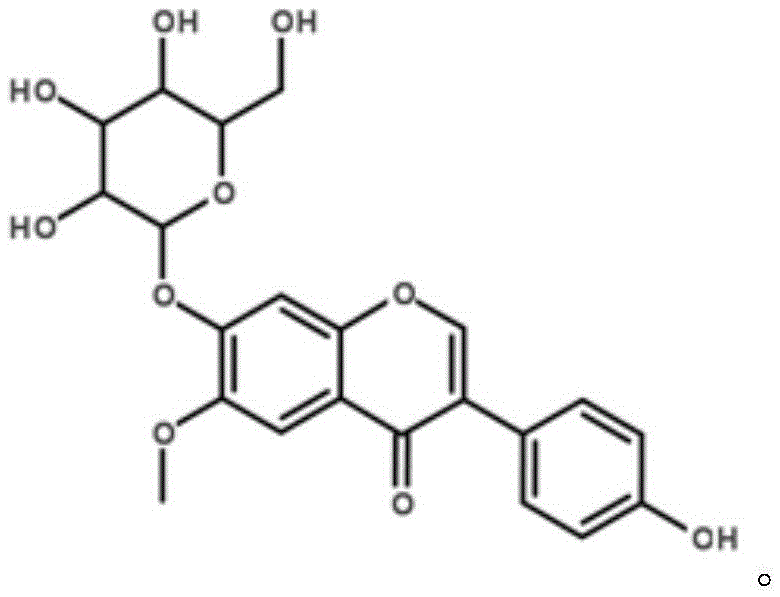
[0034] 1.8% daidzein, 0.1% daidzein, 0.8% genistin, 2.1% daidzein, 0.1% daidzein, 0.2% genistein, with soybean powder as the carrier.
[0035] Respectively take the crude drug (5.1%) and 94.9% bean powder (carrier) of the above weight ratios, and fully mix them uniformly.
[0036] 2. Efficacy test
[0037] Experimental group group:
[0038] The composition group of the present invention: the pharmaceutical composition of the present invention prepared by the aforementioned method.
[0039] Daidzin group: 5.1% daidzin, with soybean powder as carrier.
[0040] Daidzein group: 5.1% daidzein, with soybean powder as the carrier.
[0041] Genistin group: genistin 5.1%, with soybean powder as carrier.
[0042] The daidzein group: 5.1% daidzein, with soybean powder as the carrier.
[0043] Glyzein group: 5.1% glycitein, with soybean powder as the ...
Embodiment 2
[0054] Preparation and efficacy of embodiment 2 pharmaceutical composition of the present invention
[0055] 1. Preparation method
[0056] 2.2% daidzein, 0.2% daidzein, 1.2% genistin, 3.0% daidzein, 0.18% daidzein, 0.25% genistein, with soybean powder as the carrier. Total content: 7.13%.
[0057] Respectively take the bulk drug (7.13%) and 92.87% bean powder (carrier) by weight in the above proportions, and fully mix them uniformly.
[0058] 2. Efficacy test
[0059] Experimental group group:
[0060] The composition group of the present invention: the pharmaceutical composition of the present invention prepared by the aforementioned method.
[0061] Daidzin group: 7.13% daidzin, with soybean powder as carrier.
[0062] Genistin group: genistin 7.13%, with soybean powder as carrier.
[0063] The daidzein group: 7.13% daidzein, with soybean powder as the carrier.
[0064] Soybeanthin group: 7.13% glycitein, with soybean powder as the carrier.
[0065] Genistein group:...
Embodiment 3
[0074] Preparation and efficacy of embodiment 3 pharmaceutical composition of the present invention
[0075] 1. Preparation method
[0076] 2.6% daidzein, 0.3% daidzein, 1.5% genistin, 3.7% daidzein, 0.25% daidzein, 0.3% genistein, with soybean powder as the carrier. Total content: 8.65%. Respectively take the crude drug (8.65%) and 91.35% bean powder (carrier) of the above proportions by weight, and fully mix them uniformly.
[0077] 2. Efficacy test
[0078] Experimental group group:
[0079] The composition group of the present invention: the pharmaceutical composition of the present invention prepared by the aforementioned method.
[0080] Daidzin group: 8.65% daidzin, with soybean powder as carrier.
[0081] Genistin group: 8.65% genistin, with soybean powder as carrier.
[0082] The daidzein group: 8.65% daidzein, with soybean powder as the carrier.
[0083] Soybeanthin group: 8.65% glycitein, with soybean powder as the carrier.
[0084] Genistein group: 8.65% ge...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com