Separating a solvent from a nickel catalyst by distillation
一种催化剂、蒸发溶剂的技术,应用在催化剂至少部分分离领域,能够解决催化剂毒物、产率损失、不利催化剂效率等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0379] Example 1: Use of cis-2-pentenenitrile in simulated distillation.
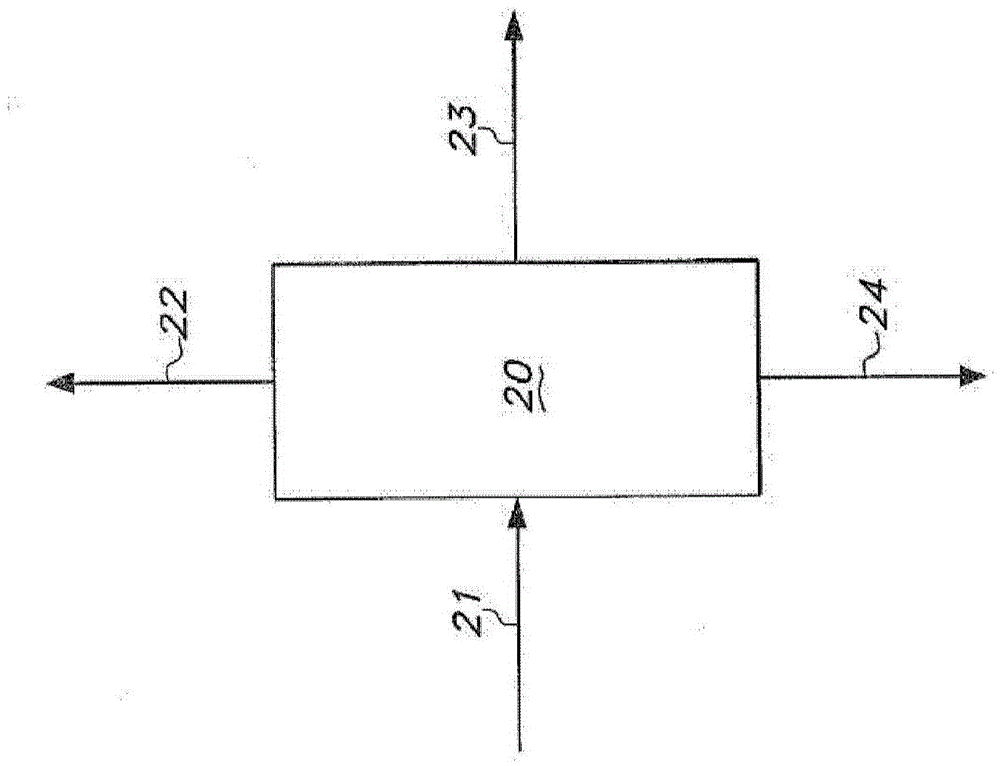
[0380] Four reaction mixtures of pentenenitrile and hydrocyanation catalyst were prepared to simulate the composition of the distillation column bottoms in the distillation column used to separate the extraction solvent from the catalyst extracted into the solvent. The catalyst is a complex of zerovalent nickel with a bidentate phosphite ligand of the formula:
[0381]
[0382] Each reaction mixture included approximately 51 wt. % trans-3-pentenenitrile, 0.4 wt. % 2-methyl-3-butenenitrile, and 575 ppm nickel. The weight ratio of nickel to bidentate phosphite ligand is 0.017.
[0383] Various amounts of cis-2-pentenenitrile were included in each reaction mixture. The first reaction mixture included an amount of cis-2-pentenenitrile such that the ratio of trans-3-pentenenitrile to cis-2-pentenenitrile (T3PN / C2PN) was 3. The second reaction mixture included an amount of cis-2-pentenenitrile such that...
Embodiment 2
[0386] Example 2: Use of trans-2-pentenenitrile in simulated distillation.
[0387] Example 1 was repeated except that trans-2-pentenenitrile was substituted for cis-2-pentenenitrile and at least some of the ratio of trans-3-pentenenitrile to 2-pentenenitrile was changed . Specifically, the first reaction mixture included an amount of trans-2-pentenenitrile such that the ratio of trans-3-pentenenitrile to trans-2-pentenenitrile (T3PN / T2PN) was 2.6. The second reaction mixture included an amount of trans-2-pentenenitrile such that the ratio of trans-3-pentenenitrile to trans-2-pentenenitrile (T3PN / T2PN) was 4.5. The third reaction mixture included an amount of trans-2-pentenenitrile such that the ratio of trans-3-pentenenitrile to trans-2-pentenenitrile (T3PN / T2PN) was 7. The fourth reaction mixture included an amount of trans-2-pentenenitrile such that the ratio of trans-3-pentenenitrile to trans-2-pentenenitrile (T3PN / T2PN) was 15.
[0388] As in the procedure of Example 1...
Embodiment 4
[0394] Example 4: Inhibition of the formation of 2M3BN in a distillation column.
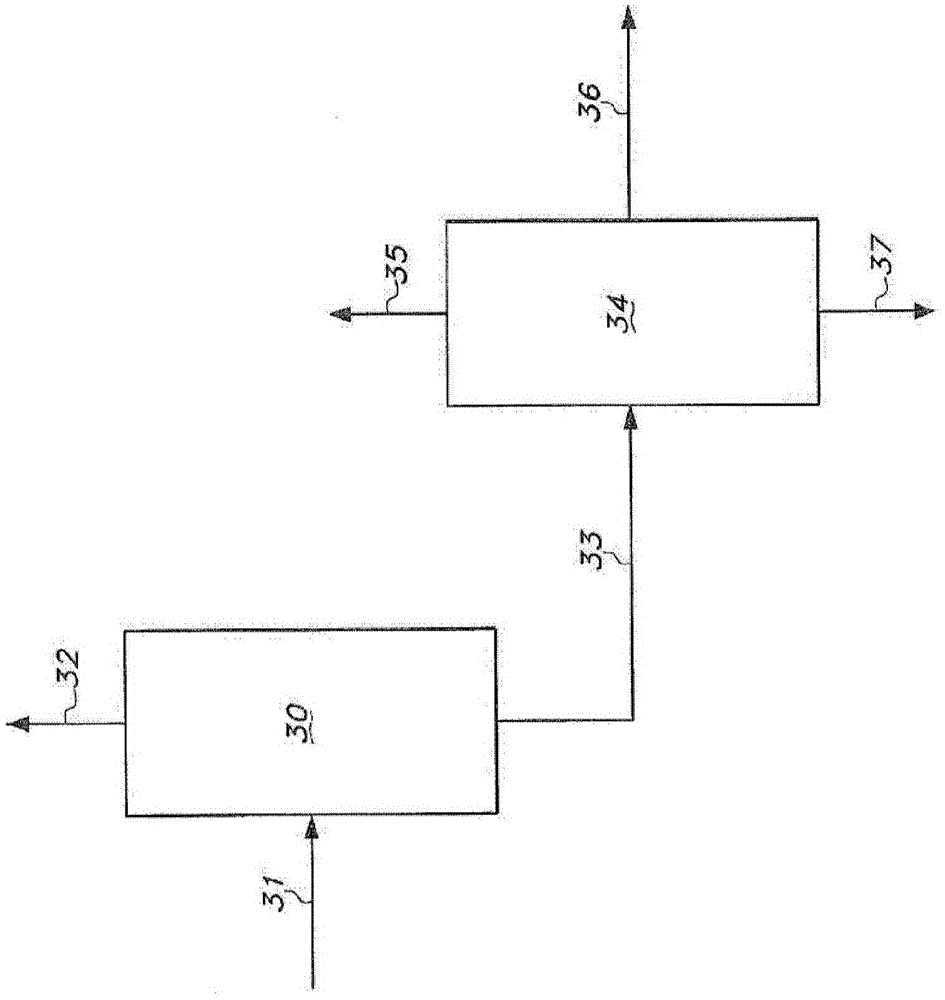
[0395] This example demonstrates the suppression of 2M3BN formation in a distillation column during the continuous production of adiponitrile.
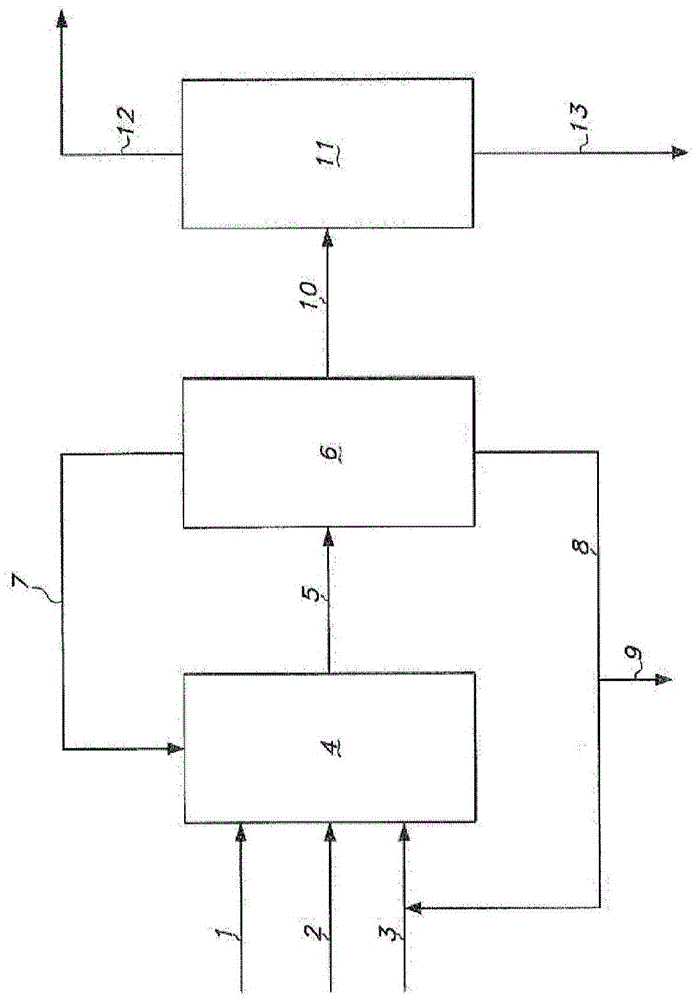
[0396] Hydrogen cyanide, 3-pentenenitrile, and a catalyst solution are continuously fed into the hydrocyanation reactor to continuously produce adiponitrile. The catalyst solution contained zerovalent nickel and a bidentate phosphite ligand of the formula shown in Example 1 . The reaction product effluent from the hydrocyanation reactor was then extracted with cyclohexane to form a light phase and a heavy phase. The light phase contained cyclohexane, catalyst and a portion of residual pentenenitriles. The heavy phase contains adiponitrile, a portion of residual pentenenitriles, and catalyst degradation products.
[0397] The light phase is continuously distilled in a distillation column including a reboiler. The fluid in the reboiler containing cata...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com