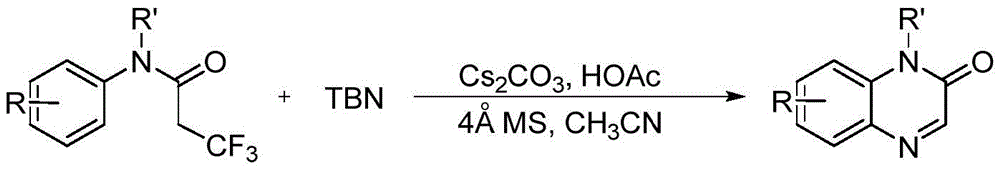Preparation method of quinoxalin-2-one derivative and product purification method
A derivative, quinoxalone technology, applied in the field of organic compound synthesis, can solve the problems of high cost, expensive, unfavorable industrialization, etc., and achieve the effect of low cost, high yield, and cheap synthesis system
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0028] The preparation method of quinoxalone derivatives comprises the following steps: taking N-methyl-N-phenyl trifluoropropionamide or its derivatives as substrate, adding TBN in the substrate, the mol ratio of substrate and TBN is 1:4; and the amount of further TBN is 4 equivalents; then dissolve with acetonitrile solution as solvent, and in molecular sieve, The amount of molecular sieve is 100 mg; and in the base Cs 2 CO 3 / acid HOAc in an air atmosphere, the preferred Cs 2 CO 3 The amount of HOAc / HOAc is 2 equivalents; the crude product is obtained by heating at 100°C for 24 hours, and its chemical reaction formula is as follows:
[0029]
[0030] Among them, R in the general formula of the substrate is one of methyl, methoxy, acetyl, nitro, fluorine, chlorine, bromine, hydrogen; R' is methyl, ethyl, isopropyl, phenyl One of , benzyl and hydrogen.
[0031] After the reaction finishes cooling, the crude product obtained by the above method is purified and purif...
specific Embodiment 1
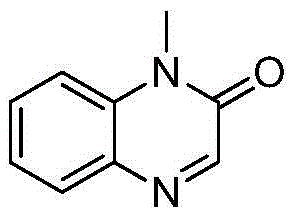
[0037] Specific embodiment one: 43.4 milligrams (0.2mmol) N-methyl-N-phenyltrifluoropropionamide, 82.4 milligrams (0.8mmol) TBN, 130.4 milligrams (0.4mmol) Cs 2 CO 3 , 24 mg (0.4 mmol) HOAc, 100 mg Molecular sieves were added to the reaction test tube, and then 2mL CH 3 CN, heated at 100°C for 24 hours, cooled after the reaction, filtered, and the filtrate was deacidified to obtain a crude product. The crude product was chromatographed on a silica gel column, washed with a mixed eluent prepared with ethyl acetate and petroleum ether, and detected by TLC , Combine the effluents containing the product, distill off the solvent with a rotary evaporator, and dry in vacuo to obtain 26.6 mg of 1-methylquinoxalin-2-one as a yellow liquid, with a yield of 83%. 1 HNMR (500MHz, CDCl 3 )δ7.45-7.39(m,3H),7.25-7.22(m,2H),3.29(s,3H); 13 CNMR (125MHz, CDCl 3 )δ144.5, 138.9, 130.1, 129.7, 128.2, 127.0, 124.9, 110.6, 36.7; LRMS (EI, 70eV) m / z (%): 160 (M + ,100),132(36),106(15),91(50),77...
specific Embodiment 2
[0039] Specific example two: 47 mg (0.2 mmol) N-methyl-N-(2-fluorophenyl) trifluoropropionamide, 82.4 mg (0.8 mmol) TBN, 130.4 mg (0.4 mmol) Cs 2 CO 3 , 24 mg (0.4 mmol) HOAc, 100 mg Molecular sieves were added to the reaction test tube, and then 2mL CH 3 CN, heated at 100°C for 24 hours, cooled after the reaction, filtered, and the filtrate was deacidified to obtain a crude product. The crude product was chromatographed on a silica gel column, washed with a mixed eluent prepared with ethyl acetate and petroleum ether, and detected by TLC , combined the effluents containing the product, distilled off the solvent with a rotary evaporator, and dried in vacuo to obtain 34.2 mg of 1-methyl-7-fluoroquinoxalin-2-one as a yellow solid, with a yield of 96%. 1 HNMR (500MHz, CDCl 3 )δ7.52-7.47(m,1H),7.38-7.35(m,1H),7.30-7.25(m,2H),3.34(s,3H); 13 CNMR (125MHz, CDCl 3 )δ158.2 (d, J=250.6Hz), 144.9, 131.9, 129.5, 127.4, 125.5, 117.3 (d, J=19.4Hz), 110.2, 36.1; LRMS (EI, 70eV) m / z (%)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com