Method for preparing peptide fragments, kit for preparing peptide fragments to be used therein, and analysis method
A peptide fragment and kit technology, which is applied in the preparation methods of peptides, the preparation of test samples, biochemical equipment and methods, etc. Analysis accuracy, effect of simplified condition setting
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
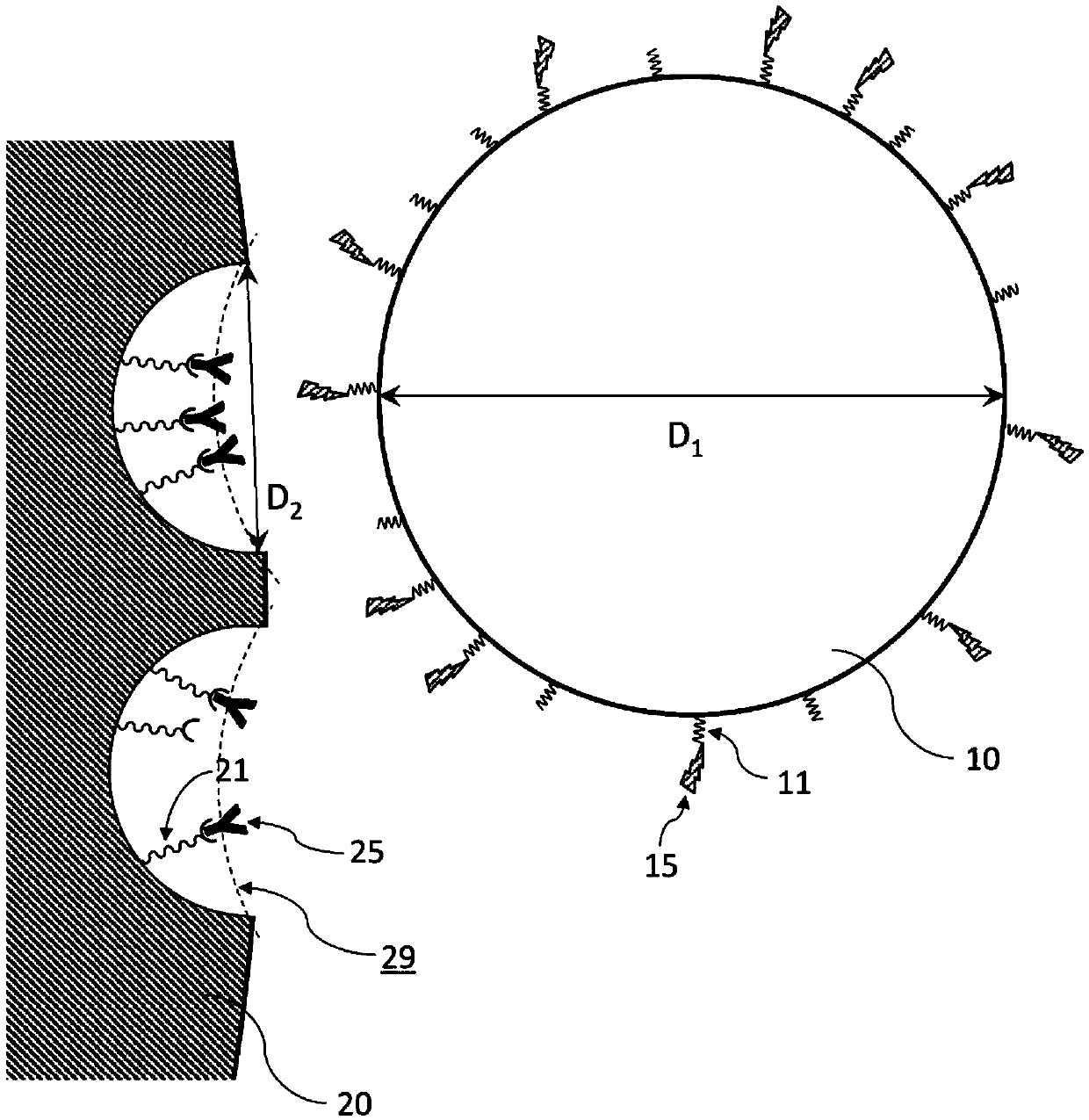
[0055] In the method for producing a peptide fragment of the present invention, a matrix protein to be cleaved is immobilized in pores of a porous body, and the porous body immobilized with the matrix protein is brought into contact with microparticles having protease immobilized on the surface in a liquid. figure 1 It is a conceptual diagram for explaining the principle of protease digestion in this invention.
[0056] In particles 10 (average particle size D 1 ) with protease 15 immobilized on the surface. The porous body 20 has a plurality of pores 29 (average pore diameter D 2 ), and matrix protein 25 is immobilized in the pores. Thus, in the method of the present invention, both the protease 15 and the matrix protein 25 are immobilized on the solid phase as minute domains, and protease digestion is performed by contacting the solid phases with each other.
[0057] Average particle diameter D of microparticles 10 1 greater than the average pore diameter D of the porou...
Embodiment
[0121] The following shows an experimental example in which human immunoglobulin G (IgG) and trastuzumab (trastuzumab (trade name: Herceptin)) were digested with protease according to the method of the present invention, and the resulting peptide fragment samples were subjected to mass spectrometry. It should be noted that the present invention is not limited to the following examples.
[0122] Hereinafter, description of % means % by weight unless otherwise specified. Reagents and the like used in this experimental example are as follows.
[0123] Trypsin (sequence grade, promega)
[0124] Lysyl endopeptidase (mass spec grade, Wako Pure Chemical Industries)
[0125] 2-Morpholineethanesulfonic acid, (MES, Doujin Chemical)
[0126] 2-[4-(2-Hydroxyethyl)-1-piperazinyl]ethanesulfonic acid (HEPES, Doujin Chemical)
[0127] Tris(hydroxymethyl)aminomethane (Tris, Wako Pure Chemical Industries)
PUM
| Property | Measurement | Unit |
|---|---|---|
| Aperture | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com