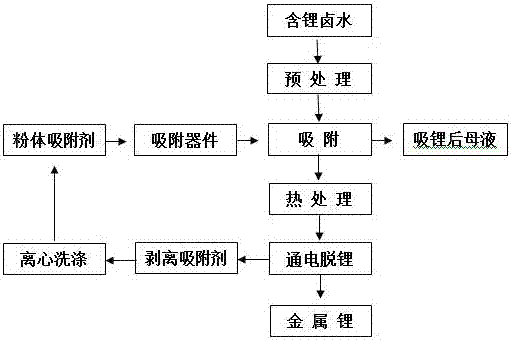
A method for extracting lithium from lithium-containing brine
A technology for extracting lithium and brine, which is applied to the improvement of process efficiency, photography technology, instruments, etc., can solve the problems of high cost and complex lithium extraction process, and achieve the effects of low cost, no need for repeated enrichment, and short process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
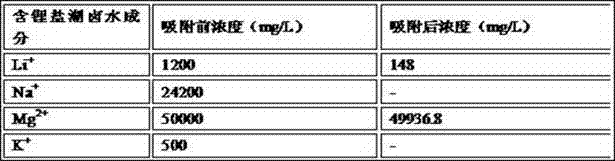
[0044] 10g of MnO with lithium intercalation activity 2 After being acidified with 0.01mol / L hydrochloric acid for 0.5h, it was loaded into the adsorption column, and 1L of lithium-containing salt lake brine was added. The composition of the lithium-containing salt lake brine is shown in Table 1. + Concentration dropped to 104mg / L, Mg 2+ Concentration dropped to 15983.7mg / L, lithium intercalation active MnO 2 to Li + The adsorption capacity is 59.6 mg / g, for Mg 2+ The adsorption capacity is 1.63 mg / g, and then the lithium-absorbed Li X mn 2 o 4 (X=0.74), after washing and drying at 120°C, heat treatment in a muffle furnace at 500°C for 3 hours;
[0045] Table 1. Lithium-containing salt lake brine composition list
[0046]
[0047] The heat-treated Li X mn 2 o 4 (X=0.74), conductive carbon black, and CMC are mixed according to the mass ratio of 94:1:5, stirred in deionized water to form a slurry, and then sprayed on a perforated aluminum strip to make a flexible el...
Embodiment 2
[0049] 5 g of FePO with lithium intercalation activity 4 After being acidified with 1mol / L sulfuric acid for 0.1h, it was loaded into the adsorption column, and 1L of lithium-containing salt lake brine was added. The composition of the lithium-containing salt lake brine is shown in Table 2. + Concentration dropped to 52mg / L, Mg 2+ The concentration is reduced to 1297.3mg / L, and lithium-intercalation active FePO 4 to Li + The adsorption capacity is 33.6 mg / g, for Mg 2+ The adsorption capacity is 0.54 mg / g, and then the lithium-absorbed Li X FePO 4 (X=0.727), after washing and drying at 80℃, in N 2 Heat treatment at 400°C for 6 hours in a muffle furnace under atmosphere;
[0050] Table 2. Lithium-containing salt lake brine composition list
[0051]
[0052] The heat-treated Li X FePO 4 (X=0.727), carbon fiber, and CMC are mixed according to the mass ratio of 80:5:15, stirred in deionized water to form a slurry, and then sprayed on foamed aluminum to make a flexible e...
Embodiment 3
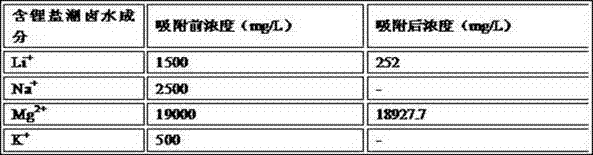
[0054] 30g of TiO with lithium intercalation activity 2 After being acidified with 0.5mol / L nitric acid for 0.3h, it was loaded into the adsorption column, and 1L of lithium-containing salt lake brine was added. The composition of the lithium-containing salt lake brine is shown in Table 3 below. + Concentration dropped to 252mg / L, Mg 2+ Concentration dropped to 18927.7mg / L, lithium intercalation active MnO 2 to Li + The adsorption capacity is 41.6mg / g, for Mg 2+ The adsorption capacity is 2.41mg / g, and then the lithium-absorbing Li 4- X Ti 5 o 12 (X=1.63), after washing and drying at 100°C, heat treatment in a muffle furnace at 600°C for 2 hours;
[0055] Table 3. Lithium-containing salt lake brine composition list
[0056]
[0057] The heat-treated Li 4- X Ti 5 o 12 (X=1.63), carbon nanotubes, and PVDF are mixed according to the mass ratio of 94.5:0.1:5.4, stirred in NMP solvent to form a slurry, and then sprayed on foamed nickel to make a flexible electrolytic...
PUM
| Property | Measurement | Unit |
|---|---|---|
| escape rate | aaaaa | aaaaa |
| escape rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com