Preparation method of alpha-beta-unsaturated carbonyl compound
A carbonyl compound and unsaturated technology, applied in the α field, can solve the problems that have not been reported in the literature, and achieve the effects of wide application range, high reaction yield and high safety performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
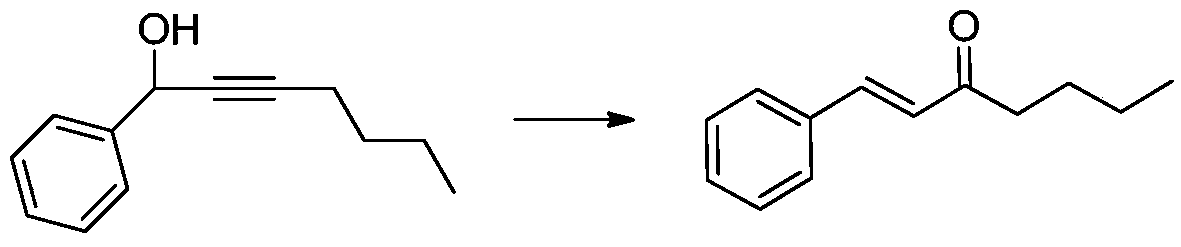
[0032] Now take 1-phenyl-2-hepten-1-ol as an example to prepare (E)-1-phenyl-1-hepten-3-one as follows:
[0033] Dissolve 1-phenyl-2-hepten-1-ol (188 mg, 1 mmol) in 5 mL of 1,2-dichloroethane, add Fe 3+ - Montmorillonite (200mg) and ethanol (5mmol, 0.29mL), stirred and heated, reacted at 80°C for 12 hours, filtered to remove Fe 3+ -Montmorillonite, concentrate the filtrate, and the concentrate is separated by column chromatography to obtain 177 mg of product (E)-1-phenyl-1-hepten-3-one, with a yield of 94%;
[0034] The reaction equation is
[0035]
[0036] 1 H-NMR (CDCl 3 ,600MHz)7.67-7.54(m,3H),7.40-7.39(m,3H),6.75(d,J=16.2Hz,1H),2.67(t,J=7.8Hz,2H),1.69-1.64(m ,2H),1.42-1.36(m,2H),0.95(t,J=7.2Hz,3H);
[0037] 13 C-NMR (CDCl 3 ,150MHz)200.6,142.3,134.6,130.3,128.9,128.2,126.3,40.7,26.5,22.4,13.9;
[0038] ESI-HRMS calculated value C 13 h 16 ONa([M+Na] + ) 211.1099, measured value 211.1092.
Embodiment 2
[0040] Now take 1-(4-methylphenyl)-2-hepten-1-alcohol as an example to prepare (E)-1-(4-methylphenyl)-1-hepten-3-one The method is implemented by the following steps:
[0041] Dissolve 1-(4-methylphenyl)-2-hepten-1-ol (202 mg, 1 mmol) in 4 mL of chlorobenzene, add Yb 3+ - Montmorillonite (100mg) and n-propanol (5mmol, 0.38mL), stirred and heated, reacted at 90°C for 8 hours, filtered to remove Yb 3+ - Montmorillonite, concentrated filtrate, the concentrate was separated by column chromatography to obtain 176 mg of product (E)-1-(4-methylphenyl)-1-hepten-3-one, with a yield of 87%;
[0042] The reaction equation is
[0043]
[0044] 1 H-NMR (CDCl 3 ,600MHz)7.53(d,J=16.2Hz,1H),7.44(d,J=8.4Hz,2H),7.19(d,J=8.4Hz,2H),6.70(d,J=16.2Hz,1H) ,2.65(t,J=7.8Hz,2H),2.37(s,3H),1.69-1.63(m,2H),1.41-1.35(m,2H),0.94(t,J=7.8Hz,3H);
[0045] 13 C-NMR (CDCl 3 ,150MHz)200.7,142.4,140.8,131.9,129.7,128.3,125.4,40.6,26.6,22.5,21.5,13.9;
[0046] ESI-HRMS calculated value C 14 h 18 ONa([...
Embodiment 3
[0048] Now take 1-(2-methylphenyl)-2-hepten-1-ol as an example to prepare (E)-1-(2-methylphenyl)-1-hepten-3-one The method is implemented by the following steps:
[0049] Dissolve 1-(2-methylphenyl)-2-hepten-1-ol (202 mg, 1 mmol) in 2 mL of p-dichlorobenzene, add Fe 3+ - Montmorillonite (100mg) and n-propanol (5mmol, 0.38mL), stirred and heated, reacted at 80°C for 24 hours, filtered to remove Fe 3+ - Montmorillonite, concentrated filtrate, the concentrate was separated by column chromatography to obtain 182 mg of product (E)-1-(2-methylphenyl)-1-hepten-3-one, with a yield of 90%;
[0050] The reaction equation is
[0051]
[0052] 1 HNMR (300MHz, CDCl 3 )7.86(d, J=16.0Hz, 1H), 7.58(d, J=7.0Hz, 1H), 7.29-7.19(m, 3H), 6.67(d, J=16.0Hz, 1H), 2.66(t, J=7.2Hz, 2H), 2.45(s, 3H), 1.73-1.63(m, 2H), 1.45-1.33(m, 2H), 0.95(t, J=7.4Hz, 3H);
[0053] 13 CNMR (75MHz, CDCl 3 ) 200.9, 140.0, 138.2, 133.7, 131.0, 130.3, 127.4, 126.6, 126.5, 41.2, 26.7, 22.7, 20.0, 14.1;
[0054] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com