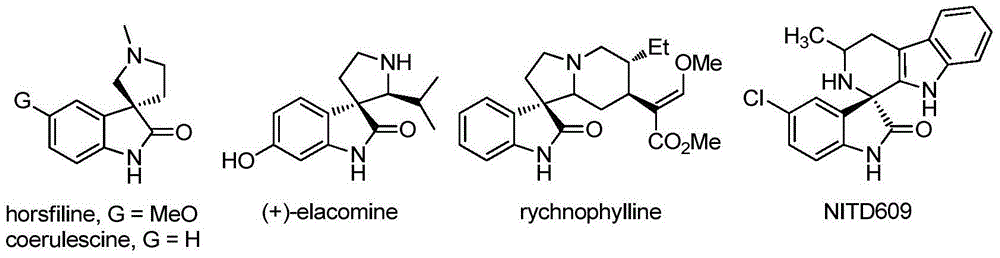
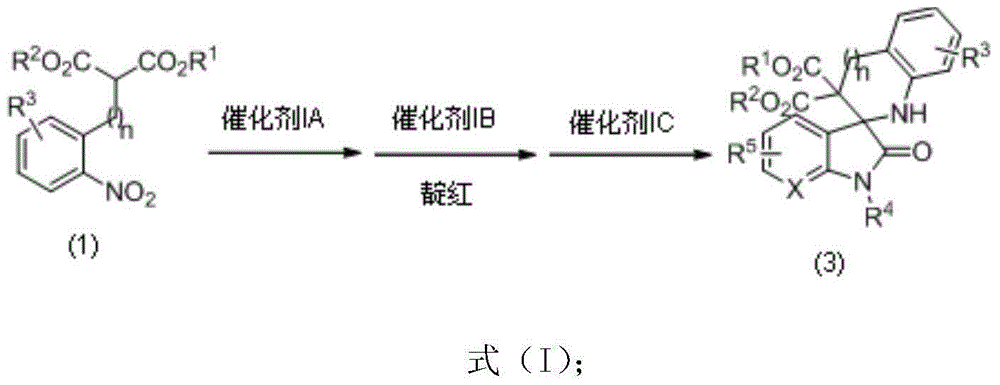
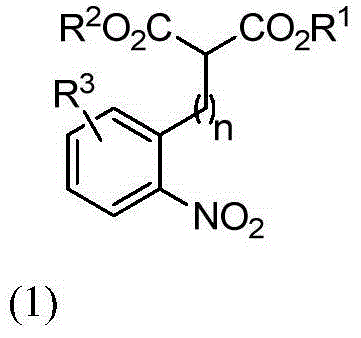
Chiral spiro-oxindole constructed by three-step relay catalysis, as well as synthesizing method and application thereof
A technology of spiro-epoxy indole and synthesis method, which is applied in organic chemistry, drug combination, anti-infective drugs, etc., can solve the problems that chiral spiro-epoxindole has not been realized and has not been reported in any literature, and achieves high Yield enantioselectivity, high yield and enantioselectivity, simple and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051]
[0052] Add 10% Pd / C (8.4mg, 10wt%), nitro compound 1a (84.4mg, 0.3mmol) and Et in the 5.0mL reaction flask successively 2 O (1mL), then stirred at room temperature under hydrogen atmosphere for 3h, then added p-TsOH (2.1mg, 4mol%), isatin 2a (58.0mg, 1.2eq) and additive MS (100mg, 120wt%), then stirred at 60°C for 6h, cooled to room temperature, first added Et 2 O (2mL), followed by chiral catalyst IC 1 (17.8mg, 10mol%), followed by stirring at 40°C for 2d, and finally direct column chromatography with eluent (petroleum ether / acetone=6:1). The target compound 3a was obtained as 100.6 mg of white solid with a yield of 85%. [α] 25 D =-90.3 (c=1.0, CHCl 3 ); 92% ee. (Chiralcel AS column, 85:15 n-hexane:isopropanol). 1 HNMR (400MHz, CDCl 3 ): δ7.63(d, J=7.6Hz, 1H), 7.34(td, J=7.6Hz, J1.2Hz, 1H), 7.23-7.17(m, 2H), 6.98-6.91(m, 2H), 6.85(d, J=7.6Hz, 1H), 6.78(d, J=8.0Hz, 1H), 4.24-4.09(m, 4H), 4.01-3.97(m, 1H), 3.19(s, 3H), 1.22 (t, J=7.2Hz, 3H), 1.03(t, J=7.2...
Embodiment 2
[0054]
[0055] Add Na to the 5.0mL reaction flask in sequence 2 S (4.7mg, 20mol%), nitro compound 1b (76.0mg, 0.3mmol) and toluene (1mL), then stirred at room temperature under hydrogen atmosphere for 3h, then added p-TsOH (2.1mg, 4mol%), isatin 2a (58.0mg, 1.2eq) and additive P 2 o 5 (100mg, 120wt%), then stirred at 40°C for 6h, cooled to room temperature, first added toluene (2mL), then added the chiral catalyst IC 4 (16.4mg, 10mol%), followed by stirring at 40°C for 2d, and finally direct column chromatography with eluent (petroleum ether / acetone=6:1). The target compound 3b was obtained as 83.5 mg of white solid with a yield of 76%. [α] 25 D =-95.3 (c=1.0, CHCl 3 ); 92% ee. (Chiralcel AD column, 80:20 n-hexane:isopropanol). 1 HNMR (400MHz, CDCl 3 ): δ7.60(d, J=7.6Hz, 1H), 7.35(td, J=7.6Hz, J=1.2Hz, 1H), 7.22(td, J=7.6Hz, J=1.2Hz, 1H), 7.13(d, J=7.6Hz, 1H), 7.00-6.92(m, 2H), 6.87(d, J=8.0Hz, 1H), 6.80(d, J=8.0Hz, 1H), 4.19(s, 1H ), 3.76(s, 3H), 3.59(s, 3H), 3...
Embodiment 3
[0057]
[0058] Add 10% Pd / C (8.4mg, 10wt%), nitro compound 1a (84.4mg, 0.3mmol) and CH 2 Cl 2 (1mL), then stirred at room temperature under hydrogen atmosphere for 3h, then added HOAc (1.8mg, 10mol%), isatin 2b (64.5mg, 1.2eq) and additive MgSO 4 (100mg, 120wt%), then stirred at 80°C for 3h, cooled to room temperature, added CH 2 Cl 2 (2mL), after adding the chiral catalyst IC 2 (18.0mg, 10mol%), followed by stirring at 40°C for 2d, and finally direct column chromatography with eluent (petroleum ether / acetone=6:1). The target compound 3c was obtained as 100.2 mg of white solid with a yield of 81%. [α] 25 D =-133.6 (c=1.0, CHCl 3 ); 92% ee. (Chiralcel AD column, 80:20 n-hexane:isopropanol). 1 HNMR (400MHz, CDCl 3 ): δ7.62(d, J=7.6Hz, 1H), 7.22(td, J=7.6Hz, J=0.8Hz, 1H), 7.05(td, J=8.4Hz, J=2.4Hz, 1H), 6.99(dd, J=8.4Hz, J=2.4Hz, 1H), 6.94(t, J=7.2Hz, 1H), 6.80-6.76(m, 2H), 4.24-4.14(m, 4H), 4.09-4.05 (m, 1H), 3.18(s, 3H), 1.23(t, J=7.2Hz, 3H), 1.09(t, J=7.2Hz, 3H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com