Synthesis method of m-phenetidine by one-pot reaction
A technology of aminophenethyl ether and m-aminophenol, which is applied in chemical instruments and methods, preparation of organic compounds, preparation of amino hydroxy compounds, etc., can solve the problems of difficult recovery of phase transfer catalysts, increase in cost, and three wastes in reduction process, etc. The effect of less pollution, convenient operation and fewer reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
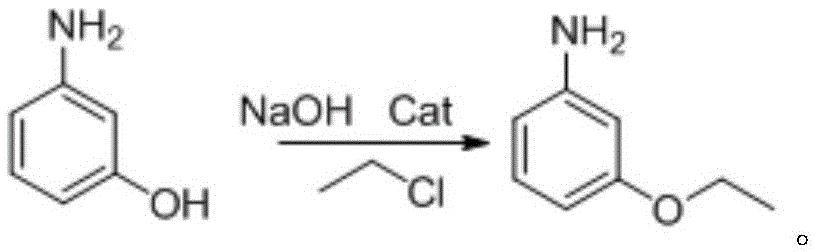
[0032] Embodiment 1, a kind of preparation method of m-aminophenetole (the method for synthesizing m-aminophenetole in one pot), take m-aminophenol as raw material, carry out the following steps successively:
[0033] (1) Put 21.8g (0.20mol) of m-aminophenol, 8.0g (0.20mol) of sodium hydroxide, and 120mL of solvent (N,N-dimethylformamide) into a 250mL reactor, and then add 0.79g (0.010mol , 5% of the molar amount of m-aminophenol) pyridine and 16.77 g (0.220 mol) ethyl chloride. After the addition, the reaction kettle was sealed and stirred at room temperature for 0.5h. Then raise the temperature to 50°C and react for 2 hours. After the reaction, cool the reaction solution to room temperature, filter the reaction solution, and keep the filtrate for use;
[0034] (2) The filtrate of step (1) gained is distilled under reduced pressure under 11mmHg vacuum degree, collects respectively the solvent of 40~55 ℃ fraction and 25.8g of light yellow oily liquid of 116~135 ℃ fraction, yi...
Embodiment 12~ Embodiment 18
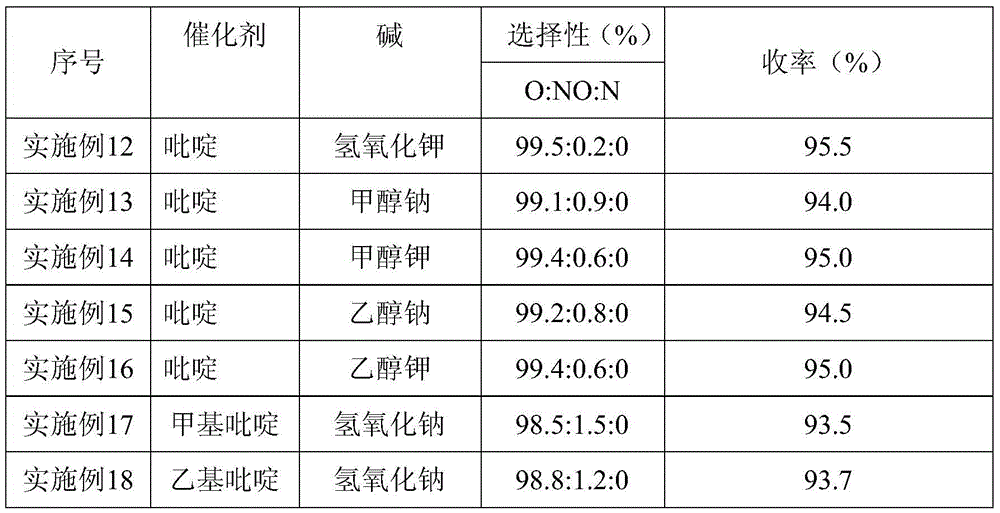
[0040] Change the name of the catalyst in Example 1, the name of the base, and the molar weight remain unchanged; the rest are the same as in Example 1, and Example 12 to Example 18 are obtained respectively. The yield and purity of gained m-aminophenetole are shown in Table 2.
[0041] Table 2
[0042]
Embodiment 19
[0043] Example 19. The solvent in Example 1 was changed from N,N-dimethylformamide to dimethyl sulfoxide, and the dosage remained unchanged; the rest were the same as in Example 1. The yield is 94.1%; m-aminophenetole: N-ethyl m-aminophenetole: N-ethyl m-aminophenol (O:NO:N)=99.0%:1.0%:0.
[0044] Comparative Example 1-1. In Example 1, "under stirring at room temperature for 0.5h" was deleted, and then "reaction at 50°C for 2h" was changed to "reaction at 50°C for 2.5h", and the rest was the same as in Example 1. The yield is 63.4%; m-aminophenetole: N-ethyl m-aminophenetole: N-ethyl m-aminophenol (O:NO:N)=87.5%:12.5%:0.
[0045] Comparative example 1-2, change the time of stirring at normal temperature in Example 1 from 0.5h to 2h; the rest is the same as Example 1. The yield is 94.1%; m-aminophenetole: N-ethyl m-aminophenetole: N-ethyl m-aminophenol (O:NO:N)=99.0%:1.0%:0.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com