Method for enzymatically synthesizing astragalin
A technology of statin glycoside and enzymatic synthesis, which is applied in the field of biomedicine, can solve the problems of increased production cost, complex synthesis process, and prolonged reaction time, and achieve the effects of reduced production cost, simple product separation, and shortened production cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
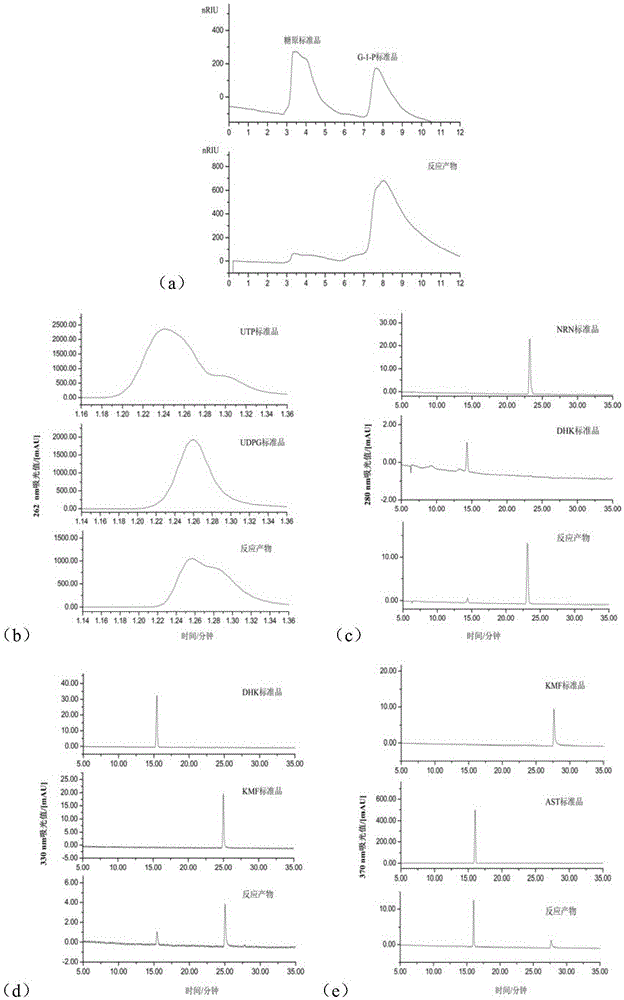
[0038] The present invention will be further described below in conjunction with the accompanying drawings and embodiments. The involved plasmids pET-32a and pGEX-5X-1 are commercial materials.
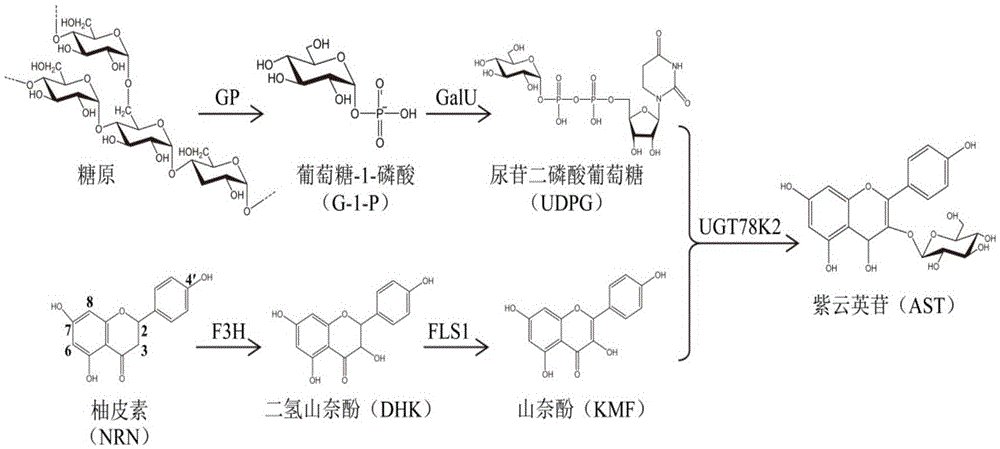
[0039] 1. Cloning, expression and purification of key enzymes in the biosynthetic pathway of ascarin;
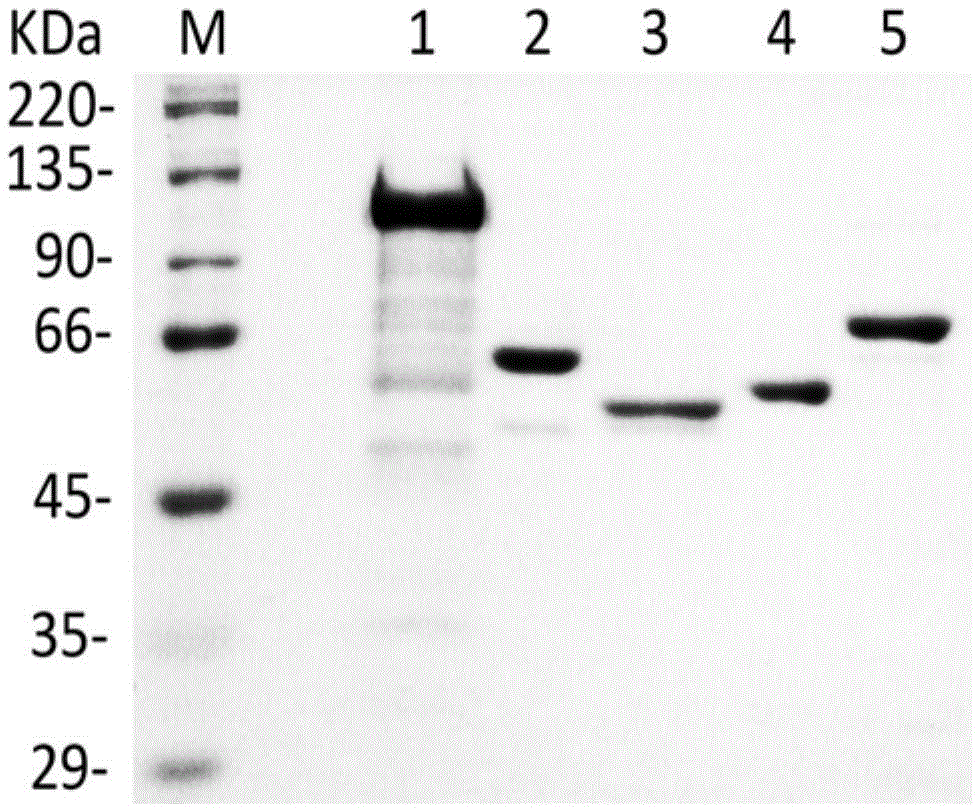
[0040] 1.1 Clone flavanone-3-hydroxylase (F3H) gene f3h and flavanone synthase (FLS1) gene fls1 from Arabidopsis thaliana, clone glycogen phosphorylase (GP) gene gp from New Zealand rabbit to prokaryotic The expression vector pET-32a was used to construct recombinant plasmids pET-32a-f3h, pET-32a-fls1 and pET-32a-gp, which were transformed into Escherichia coli BL21(DE3), induced by IPTG, and the fusion protein was purified by nickel agarose gel.
[0041] 1.2 Clone the flavonoid 3-O-glucosyltransferase (UGT78K2) gene ugt78k2 of UDP-glucose from black soybean, clone it into the plasmid pGEX-5X-1, construct the pGEX-5X-1-ugt78k2 recombinant plasmid, and transform it into Escherichia co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com