Preparation method of brexpiprazole
A compound, room temperature technology, applied in organic chemistry and other directions, can solve the problems of complex post-processing, high cost, and affecting the quality of brexpiprazole
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
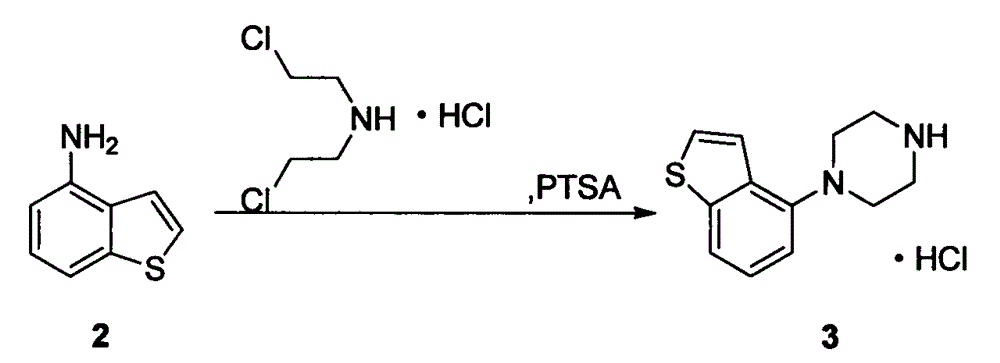
[0021] 3 preparations of 1-benzo[b]thiophen-4-yl-piperazine hydrochloride compound
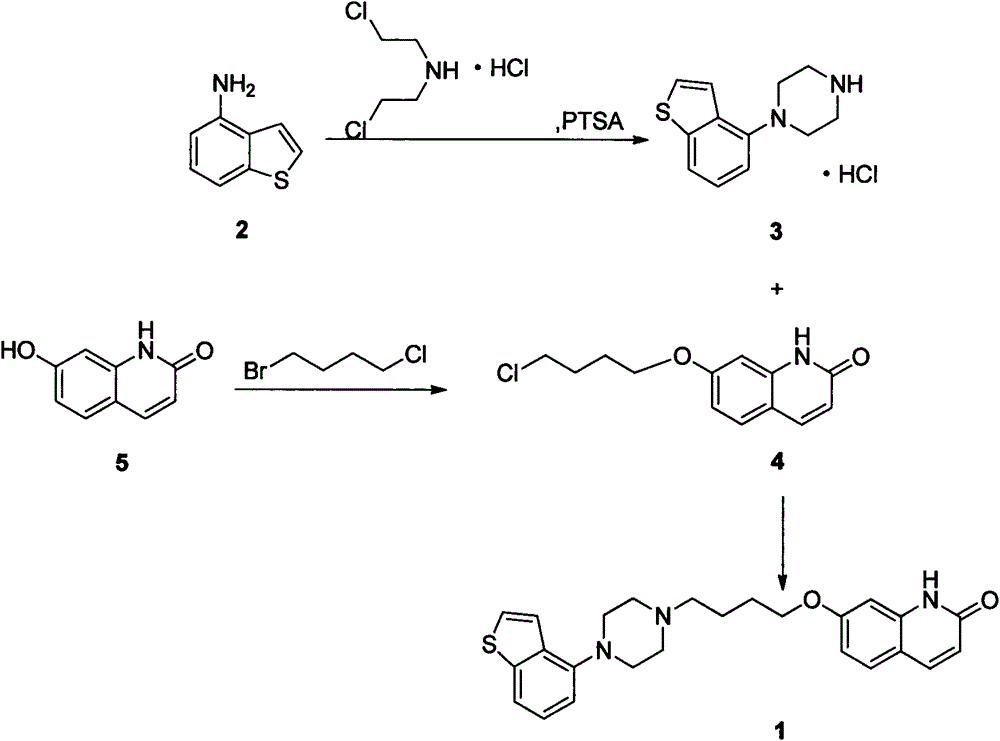
[0022] Add 14.92g (100mmol) of 4-aminobenzo[b]thiophene and 17.85g (100mmol) of bis(2-chloroethyl)amine hydrochloride into the reaction flask, then add 0.86g (5mmol) of p-toluenesulfonamide ) and xylene 225mL, stirred and reacted at 120-150°C for 16 hours. Then it was naturally lowered to room temperature, and then lowered to 0°C for 2 hours, and 21.66 g of off-white solid crystals were obtained by suction filtration, with a yield of 85.0% and a purity of 98%.
[0023] 1 H-NMR (DMSO-d 6 )δppm: 3.24-3.35 (8H, m), 6.94 (1H, d, J = 7.6Hz), 7.31 (1H, dd, J = 7.8Hz, 7.8Hz), 7.50 (1H, d, J = 5.5Hz) , 7.68 (1H, d, J=8.1Hz), 7.73 (1H, d, J=5.5Hz), 9.36 (2H, brs).
Embodiment 2
[0025] Preparation of 7-[4-(4-benzo[b]thiophen-4yl-piperazin-1-yl)butoxy]-1H-quinolin-2-one compound 1
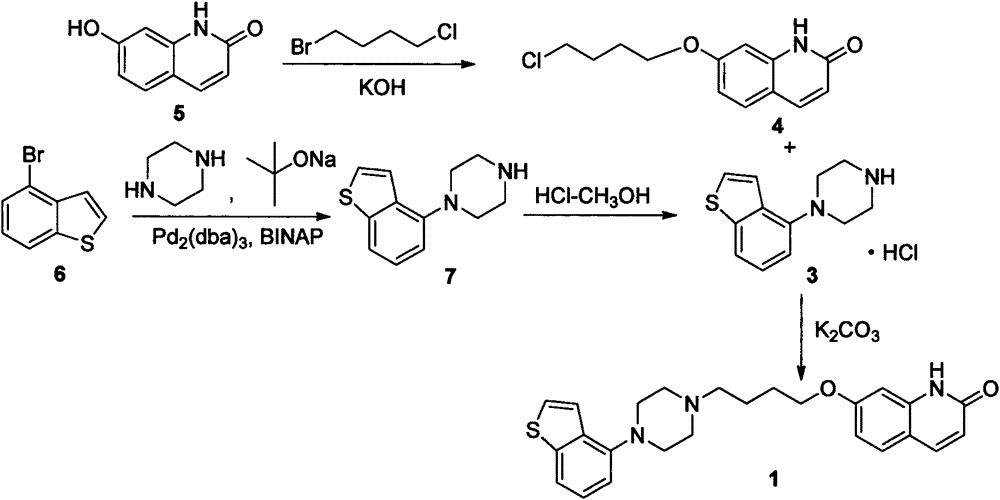
[0026] Add 14.01g (55mmol) of 1-benzo[b]thiophen-4-yl-piperazine hydrochloride, 15.90g (150mmol) of sodium carbonate, 140mL of water and 70mL of methanol into the reaction flask, heat up to 50°C and stir for 30min After the solid was dissolved, 12.58 g (50 mmol) of 7-(4-chlorobutoxy)-1H-quinolin-2-one was added, then the temperature was raised to 70° C., and the reaction was stirred for about 16 hours, then naturally cooled to room temperature, and then lowered to Heat it at 0°C for 2 hours, and filter with suction to obtain 20.70 g of off-white solid crystals, with a yield of 95.5% and a purity of 98%.
[0027] 1 H-NMR (DMSO-d 6 )δppm: 1.6-1.75 (2H, m), 1.75-1.9 (2H, m), 2.45 (2H, t, J=7Hz), 2.5-2.8 (4H, m), 2.9-3.2 (4H, m), 4.06 (2H, t, J = 6.5Hz), 6.31 (1H, d, J = 9.5Hz), 6.75-6.85 (2H, m), 6.89 (1H, d, J = 7.5Hz), 7.28 (1H, dd , J=8Hz, 8Hz), 7.40(1H, d, J=5.5Hz), 7....
Embodiment 3
[0029] 4 Preparation of 7-(4-chlorobutoxy)-1H-quinolin-2-one compound
[0030] Add 16.12g (100mmol) of 7-hydroxy-2(1H)-quinoline copper into the reaction flask, then add 160mL of methanol, then add 7.01g (125mmol) of potassium hydroxide, raise the temperature to 50°C and stir for 30min, after the solid dissolves Add 51.44 g (300 mmol) of 1-bromo-4-chlorobutane, then raise the temperature to reflux, stir and react for 16 hours, then cool down to room temperature naturally, then lower to 0-5°C and keep warm for 2 hours. g, yield 81.2%, purity 95%.
[0031] 1 H-NMR (DMSO-d 6 )δppm: 1.95-2.15 (4H, m), 3.60-3.70 (2H, m), 4.10 (2H, t, J = 5.6Hz), 6.56 (1H, dd, J = 9.0Hz, 3.8Hz), 6.82 ( 1H, dd, J=8.7Hz, 2.4Hz), 6.86 (1H, d, J=2.3Hz), 7.45 (1H, d, J=8.7Hz), 7.75 (1H, d, J=9.4Hz), 12.55 (1H, brs).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com