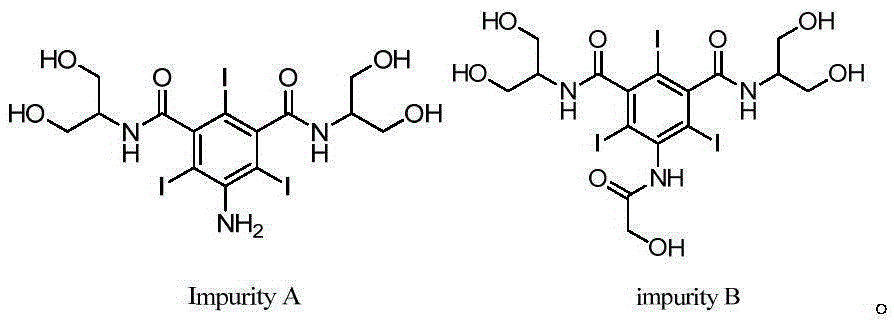
Synthetic methods of an impurity A and an impurity B of iopamidol
A synthesis method and a technology of iopamidol, applied in the field of medicine, can solve the problems of difficult control of the reaction process, poor ester solubility, difficult removal and the like, and achieve the effects of simple solvent category and simple separation and purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
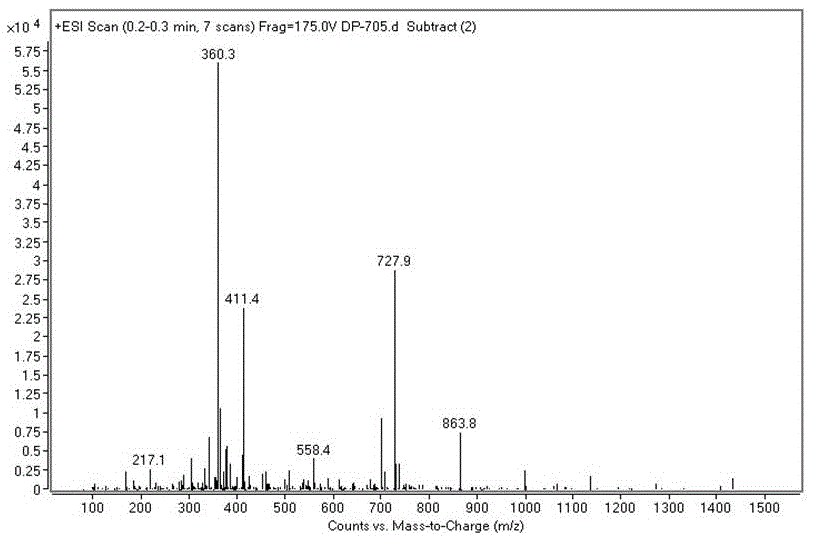
[0037] Embodiment 1: the synthesis of iopamidol impurity A
[0038] According to the mass volume ratio of 5-amino-1,3-phthaloyl chloride and N,N-dimethylacetamide is 1g:2mL, put 59.6g of 5-amino-1,3-phthaloyl chloride in the reaction bottle Add 119mL of N,N-dimethylacetamide, stir at room temperature to dissolve completely, add 32mL of triethylamine, cool to 0-5°C in an ice bath, dissolve 21g of serinol in 168mL of N,N-dimethylacetamide , slowly drop into the solution, keep the solution temperature less than 10°C, after the drop is completed, heat up to 20-30°C for reaction, TLC tracking detection (tetrahydrofuran: dichloromethane = 1:1), react for 27 hours, concentrate under reduced pressure, Cool down to room temperature, add 298 mL of absolute ethanol to the residue, heat and reflux for 2 hours, lower to room temperature and stir for 30 minutes, filter, and rinse the solid with absolute ethanol 3 times, 15 mL each time, to obtain 60.6 g of light yellow solid iodine Alcohol...
Embodiment 2
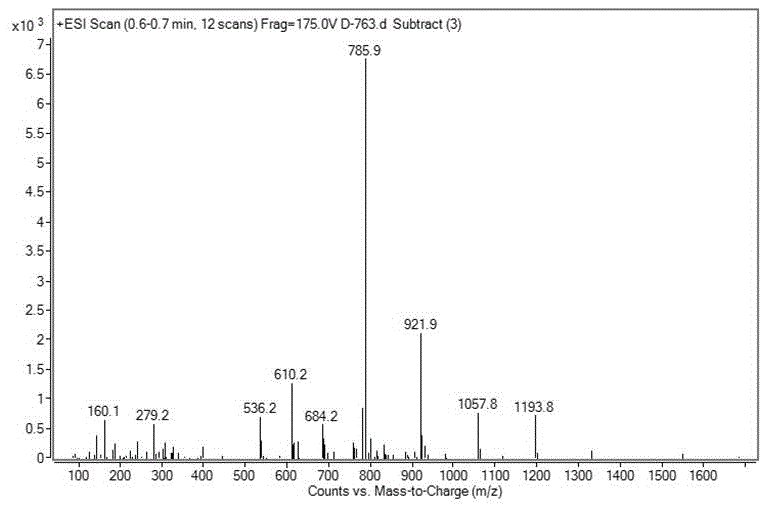
[0039] Embodiment 2: the synthesis of iopamidol impurity A
[0040] According to the mass volume ratio of 5-amino-1,3-dibenzoyl chloride and N,N-dimethylacetamide is 1g:2mL, put 90g of 5-amino-1,3-dibenzoyl chloride in 1000mL three-port reaction In the bottle, add 180mL N,N-dimethylacetamide, stir at room temperature to dissolve completely, add 48.3mL triethylamine, cool to 0-5°C in an ice bath, dissolve 31.5g serinol in 252mL N , in N-dimethylacetamide, slowly dropwise, keeping the solution temperature below 5°C, after the drop, raise the temperature to 20-30°C to react, TLC tracking detection (developing agent is tetrahydrofuran: dichloromethane = 1:1) , reacted for 26 hours, concentrated under reduced pressure, lowered to room temperature, added 450 mL of isopropanol to the residue, heated to reflux for 3 hours, lowered to room temperature and stirred for 30 minutes, filtered, and the solid was rinsed with absolute ethanol 3 times, 40 mL each time , to obtain 85.2g light y...
Embodiment 3
[0041] Embodiment 3: the synthesis of iopamidol impurity A
[0042] In the step of synthesizing impurity A in this example, triethylamine was replaced by diisopropylethylamine, and other steps were the same as in Example 1 to obtain 58.5 g of impurity A, which was higher than 95% as determined by HPLC.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com