Topiroxostat tablet and preparation method thereof
A technology of topinostat and sita tablets, applied in the field of pharmaceutical preparations, to achieve the effects of stable quality, stable and controllable process, and high feasibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
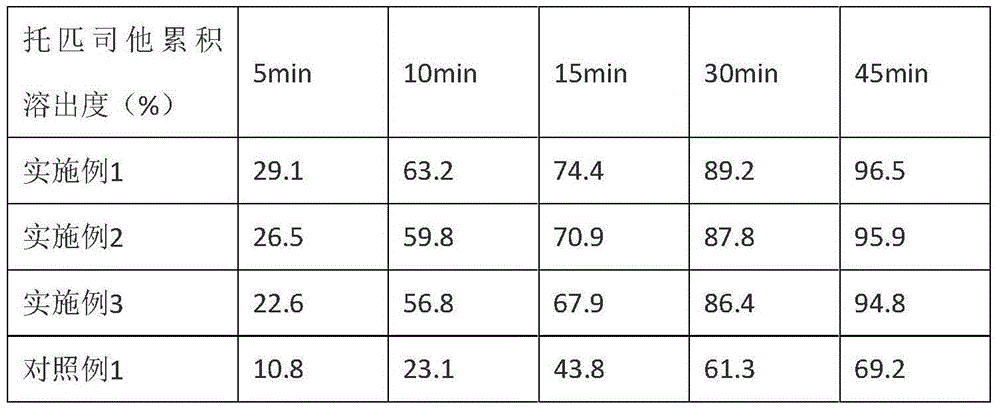
Embodiment 1
[0030] Specification: 20mg
[0031] Element
[0032] Specific process: Airflow pulverization of topinostat, control the air pressure at 0.3-0.8 MPa, make the particle size D90 less than 50 μm, weigh the micronized topinostat according to the prescription amount, and mix with the prescription amount of lactose and microcrystalline fiber Sodium croscarmellose and 1 / 2 recipe quantity were mixed through a 30-mesh sieve in a wet granulator, stirred at 600rpm for 10 minutes, added 5% hydroxypropyl cellulose aqueous solution, stirred at 600rpm, and sheared at 2000rpm to prepare grains for 2 minutes. Pass through a 24-mesh sieve for wet trimming, dry at 60°C until the moisture content is 0.5% to 4.0%, pass through a 20-mesh sieve for granulation. Add the prescription amount of magnesium stearate and 1 / 2 the prescription amount of croscarmellose sodium, and mix in a three-dimensional mixer for 5 minutes. Tablets were compressed with a 6mm round die.
Embodiment 2
[0034] Specification: 40mg
[0035] Element
[0036] Specific process: Airflow pulverization of topinostat, control the air pressure at 0.3-0.8 MPa, make the particle size D90 less than 50 μm, weigh the micronized topinostat according to the prescription amount, and mix with the prescription amount of lactose and microcrystalline fiber Sodium croscarmellose and 1 / 2 recipe quantity were mixed through a 30-mesh sieve in a wet granulator, stirred at 600rpm for 10 minutes, added 5% hydroxypropyl cellulose aqueous solution, stirred at 600rpm, and sheared at 2000rpm to prepare grains for 2 minutes. Pass through a 24-mesh sieve for wet trimming, dry at 60°C until the moisture content is 0.5% to 4.0%, pass through a 20-mesh sieve for granulation. Add the prescription amount of magnesium stearate and 1 / 2 the prescription amount of croscarmellose sodium, and mix in a three-dimensional mixer for 5 minutes. Tablets were compressed with a 7mm round die.
Embodiment 3
[0038] Specification: 60mg
[0039] Element
[0040] lactose
[0041] Specific process: Airflow pulverization of topinostat, control the air pressure at 0.3-0.8 MPa, make the particle size D90 less than 50 μm, weigh the micronized topinostat according to the prescription amount, and mix with the prescription amount of lactose and microcrystalline fiber Sodium croscarmellose and 1 / 2 recipe quantity were mixed through a 30-mesh sieve in a wet granulator, stirred at 600rpm for 10 minutes, added 5% hydroxypropyl cellulose aqueous solution, stirred at 600rpm, and sheared at 2000rpm to prepare grains for 2 minutes. Pass through a 24-mesh sieve for wet trimming, dry at 60°C until the moisture content is 0.5% to 4.0%, pass through a 20-mesh sieve for granulation. Add the prescription amount of magnesium stearate and 1 / 2 the prescription amount of croscarmellose sodium, and mix in a three-dimensional mixer for 5 minutes. Tablets were pressed with an 8mm round die...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Granularity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com