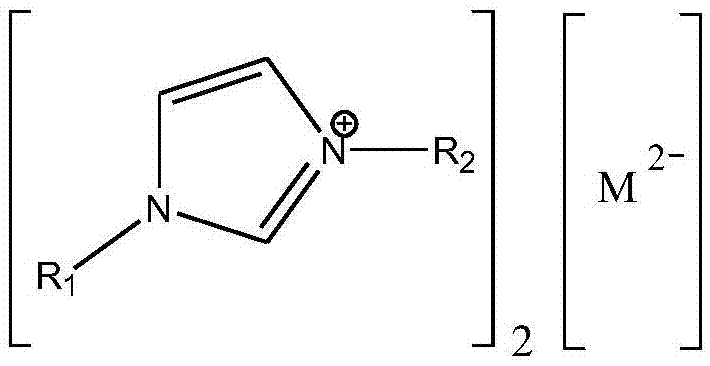
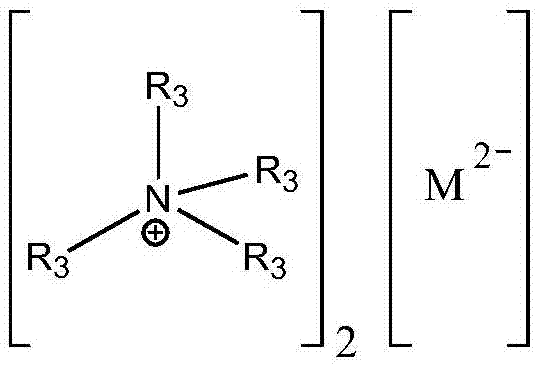
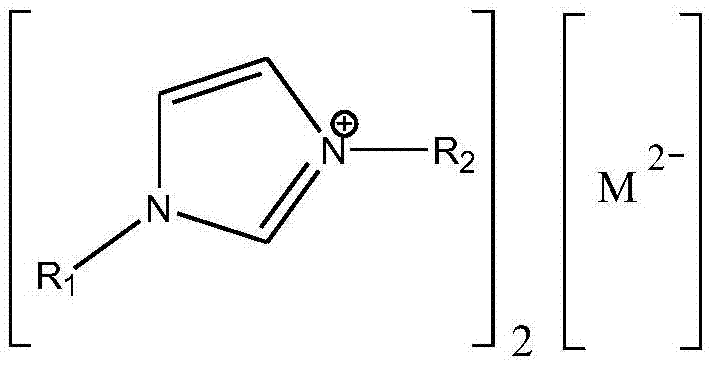
Method for preparing didodecyl carbonate by taking oxometallate ion liquid as catalyst
An ionic liquid, dimethyl carbonate technology, applied in the preparation of organic carbonate, organic chemistry and other directions, can solve the problems of complex preparation process of solid base catalyst, poor catalyst reaction activity, harsh reaction conditions, etc., to achieve mild reaction conditions, synthetic Simple method and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] In the reaction kettle, 18g (0.2mol) of dimethyl carbonate, 111.75g (0.6mol) of lauryl alcohol and 0.649g (accounting for 0.5% of the total mass of raw materials) of 1-ethyl-3-methylimidazolium tungstate ionic liquid were successively added. wt%), stirred and heated to a reaction temperature of 90° C., reacted at a constant temperature for 3 hours, and analyzed by gas chromatography. The conversion rate of dimethyl carbonate was 95%, and the yield of dilauryl carbonate was 90%.
Embodiment 2
[0021] In the reactor, add dimethyl carbonate 18g (0.2mol), lauryl alcohol 74.5g (0.4mol) and 1-propyl-3-methylimidazolium tungstate ionic liquid 1.85g (accounting for 2wt of the total mass of raw materials) successively. %), stirred and heated to a reaction temperature of 100° C., reacted at a constant temperature for 3 hours, and analyzed by gas chromatography. The conversion rate of dimethyl carbonate was 96%, and the yield of dilauryl carbonate was 92%.
Embodiment 3
[0023] In the reactor, add dimethyl carbonate 18g (0.2mol), lauryl alcohol 74.5g (0.4mol) and 1-butyl-3-methylimidazolium tungstate ionic liquid 0.925g (accounting for 1wt of the total mass of raw materials) successively. %), stirred and heated to a reaction temperature of 110° C., reacted at a constant temperature for 2 hours, and analyzed by gas chromatography. The conversion rate of dimethyl carbonate was 99%, and the yield of dilauryl carbonate was 97%.
[0024] After the reaction, reclaim the 1-butyl-3-methylimidazolium tungstate ionic liquid catalyst, and carry out 10 cycle experiments successively according to the above reaction conditions, and the conversion rate of dimethyl carbonate detected by gas chromatography remains at 98% all the time ~99%, the yield of dilauryl carbonate is maintained at 96% ~ 97%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com