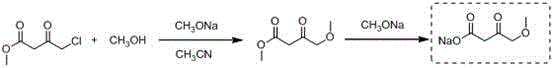
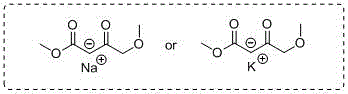
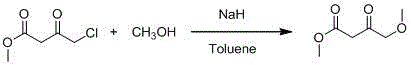Synthetic method of methyl 4-methoxyacetoacetate
A technology of methyl methoxy acetoacetate and a synthesis method, applied in the field of synthesis of methyl 4-methoxy acetoacetate, can solve problems such as expensive and unaffordable molecular distillation equipment, achieve safe use and reduce usage , saving and using simple effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Add 250mL tetrahydrofuran to the 2L reaction bottle in advance, and start stirring under the protection of argon. At this time, the internal temperature is 25°C, and 30g (0.74mol) sodium hydride (containing 40wt% Mineral oil) and 50g (0.74mol) of potassium methylate, after the addition, continue to add 450mL of tetrahydrofuran; slowly drop a mixed solution of 30g of methanol and 100g (0.67mol) of methyl 4-chloroacetoacetate at an internal temperature of 20°C, Adjust the stirring rate at about 310 rpm, and finish adding in about 4 hours; the internal temperature rises to 20-25°C and stirs for 5 hours, TLC detects that the raw materials have reacted completely, and the system begins to cool down. It can be seen that the color of the solution is light yellow and a large amount of solids are suspended. Add 200mL of tetrahydrofuran at 11°C, and when the internal temperature drops to -2°C, slowly add 100mL of hydrochloric acid solution with a molar concentration of 2mol / L (pre...
Embodiment 2
[0025] Add 250mL tetrahydrofuran to the 2L reaction bottle in advance, and start stirring under the protection of argon. At this time, the internal temperature is 25°C, and 30g (0.74mol) sodium hydride (containing 40wt% mineral Oil) and 50g (0.74mol) of potassium methylate, after the addition, continue to add 450mL of tetrahydrofuran; slowly add a mixed solution of 30g of methanol and 100g (0.67mol) of methyl 4-chloroacetoacetate dropwise at an internal temperature of 20°C to adjust The stirring rate is around 310 rpm, and the addition is completed in about 4 hours; the internal temperature rises to 20-25°C and stirs for 5 hours, and TLC detects that the raw materials have completely reacted; Add 200mL of tetrahydrofuran at 11°C, and when the internal temperature drops to -2°C, slowly add 100mL of hydrochloric acid solution with a molar concentration of 2mol / L (pre-cooled to 0-5°C), and keep the internal temperature below 0°C. After adding in about 20 minutes, the pH of the re...
Embodiment 3
[0027]Add 250mL tetrahydrofuran to the 2L reaction bottle in advance, and start stirring under the protection of argon. At this time, the internal temperature is 25°C, and 30g (0.74mol) sodium hydride (containing 40wt% mineral oil) and 50g (0.74mol) of potassium methylate, after the addition, continue to add 450mL of tetrahydrofuran, and slowly add a mixed solution of 30g of methanol and 100g (0.67mol) of methyl 4-chloroacetoacetate dropwise at an internal temperature of 20°C to adjust The stirring rate is around 310 rpm, and the addition is completed in about 4 hours; the internal temperature rises to 20-25°C and stirs for 5 hours, and TLC detects that the raw materials have completely reacted; Add 200mL of tetrahydrofuran at 11°C, and when the internal temperature drops to -2°C, slowly add 100mL of hydrochloric acid solution with a molar concentration of 2mol / L (pre-cooled to 0-5°C), and keep the internal temperature below 0°C. After adding in about 20 minutes, the pH of the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com