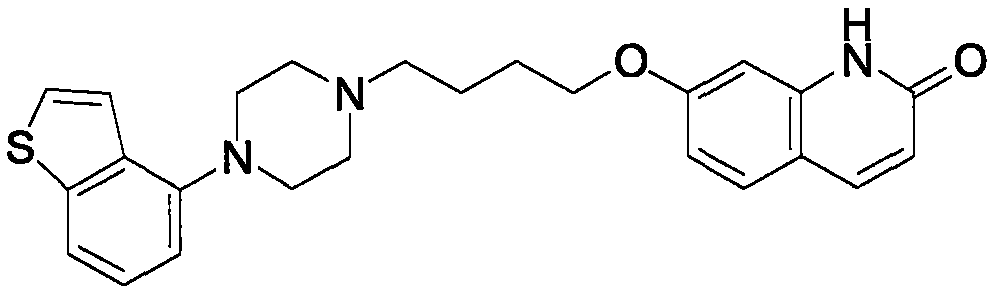
Brexpiprazole orally disintegrating tablets
A technology for oral disintegrating tablets and epipiprazole, which is applied in the field of epipiprazole oral disintegrating tablets, can solve the problems of difficulty in covering up the irritation of epipiprazole, the preparation is easily broken, and is uncomfortable for transportation, and achieves enhanced medication administration. The effect of compliance, improved bioavailability, improved taste
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
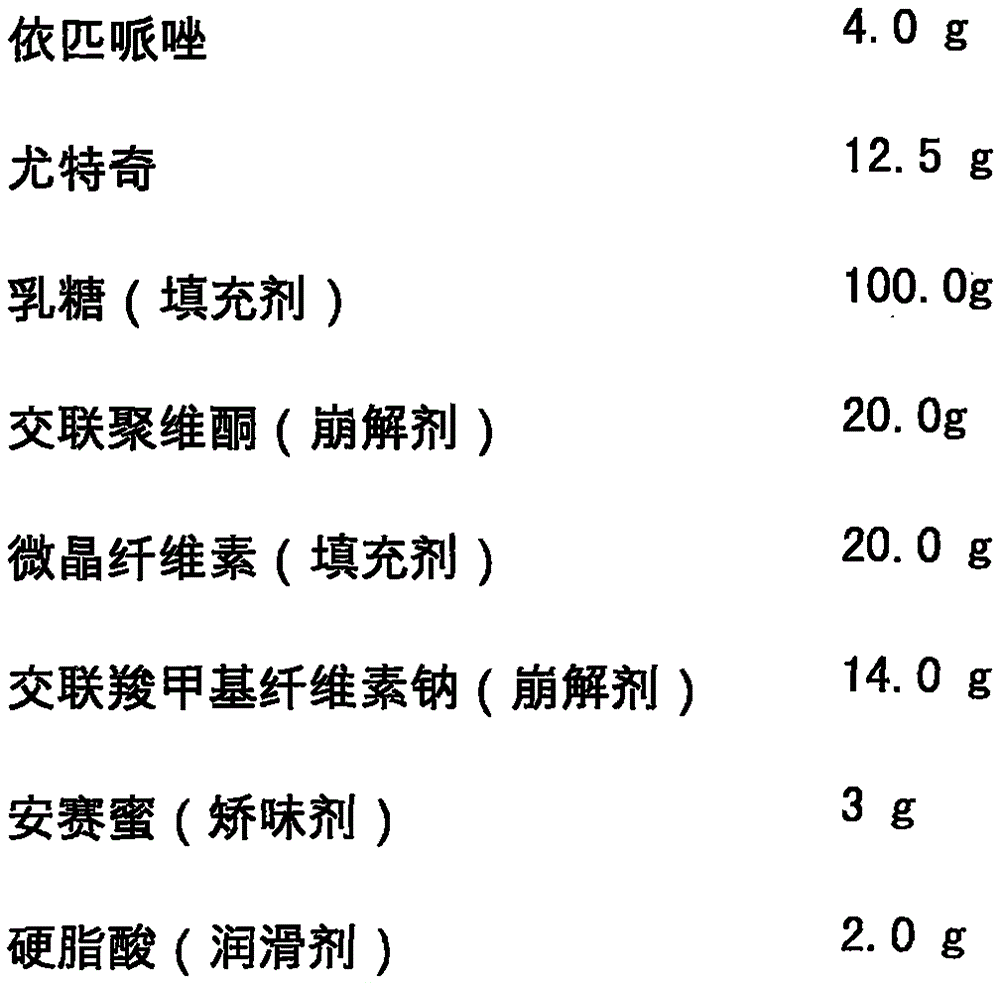
[0020] Epipiprazole Orally Disintegrating Tablets is formulated with the following components, and the dosage is 1000 tablets:
[0021]
[0022]
[0023] Its preparation method comprises the following steps:
[0024] The epipiprazole and the prescription amount of lactose are micronized until the particle size of the raw material is less than 10 μm;
[0025] The micronized raw material and lactose co-powder and the prescription amount of Udraki are mixed evenly;
[0026] 3) The diluent, disintegrant and lubricant are gradually added by an equal amount incremental method, after repeated sieving and mixing, alcohol water is used as a wetting agent, granulated, and dried after external pressure tablet to obtain the oral disintegration of epipiprazole. Unpack.
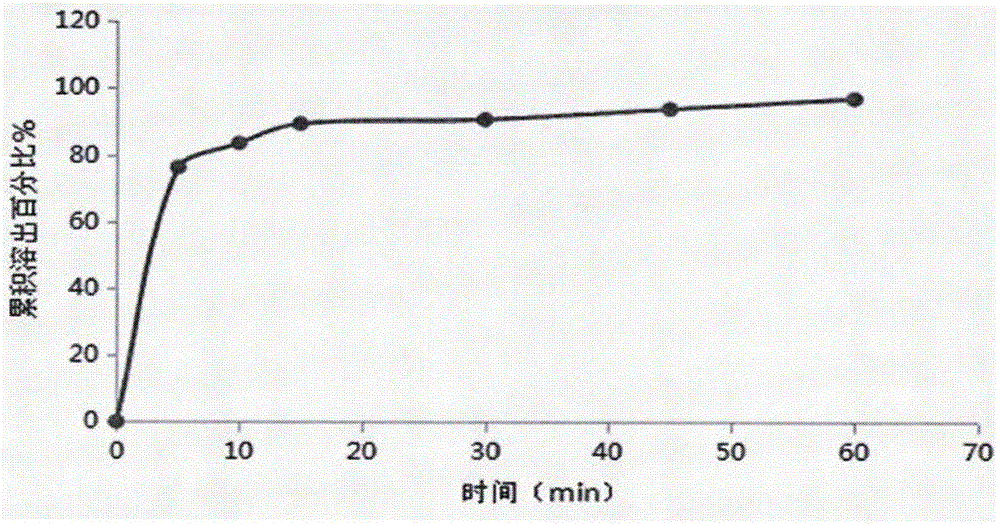
[0027] 4) Detect the dissolution curve of the prepared orally disintegrating tablet, and it should reach more than 85% in 30 minutes.
[0028] test results
[0029] Mouthfeel: Slightly bitter, but acceptable.
...
Embodiment 2
[0036] Epipiprazole Orally Disintegrating Tablets is formulated with the following components, and the dosage is 1000 tablets:
[0037]
[0038]
[0039] Its preparation method comprises the following steps:
[0040] 1) Micronize epipiprazole and the recipe amount of lactose to the particle size of the raw material <10 μm;
[0041] 2) The micronized raw material and lactose co-powder and the recipe amount are utech, and mix them evenly;
[0042] 3) The diluent, disintegrant and lubricant are gradually added by an equal amount incremental method, after repeated sieving and mixing, alcohol water is used as a wetting agent, granulated, and dried after external pressure tablet to obtain the oral disintegration of epipiprazole. Unpack.
[0043] 4) Detect the dissolution curve of the prepared orally disintegrating tablet, and it should reach more than 85% in 30 minutes.
[0044] test results
[0045] Mouthfeel: No bitterness and numbness, slightly sweet and slightly cool. ...
Embodiment 3
[0052] Epipiprazole Orally Disintegrating Tablets is formulated with the following components, and the dosage is 1000 tablets:
[0053]
[0054]
[0055] Its preparation method comprises the following steps:
[0056] 1) Micronize epipiprazole and the recipe amount of lactose to the particle size of the raw material <10 μm;
[0057] 2) The micronized raw material and lactose co-powder and the recipe amount are utech, and mix them evenly;
[0058] 3) The diluent, disintegrant and lubricant are gradually added by an equal amount incremental method, after repeated sieving and mixing, alcohol water is used as a wetting agent, granulated, and dried after external pressure tablet to obtain the oral disintegration of epipiprazole. Unpack.
[0059] 4) Detect the dissolution curve of the prepared orally disintegrating tablet, and it should reach more than 85% in 30 minutes.
[0060] test results
[0061] Mouthfeel: No bitterness, coolness.
[0062] Compressibility: good
[0...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com