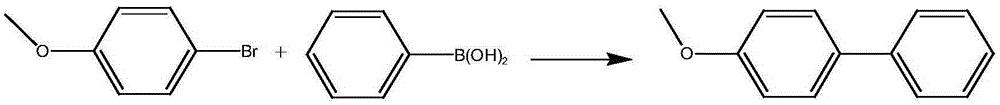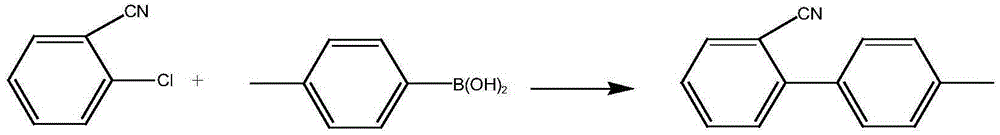Metal palladium catalyst, and preparation method and application thereof
A technology of metal palladium and catalyst, applied in the field of metal palladium catalyst and preparation thereof, can solve the problems of difficult recovery and use of catalyst, low usage amount of palladium catalyst, low catalyst activity, etc., and achieves great application value, simple and convenient post-processing, and high catalytic performance. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment example 1
[0020] Under argon, palladium acetate (1.2 mg, 0.005 mmol) and DIPPF (3.2 mg, 0.0075 mmol) were added to a Schlinker tube containing 2 mL of toluene, stirred for 5 min, and tris(4-bromophenyl)amine was added (120.5mg, 0.25mmol), two-1-adamantylphosphine (75.6mg, 0.25mmol) and sodium tert-butoxide (36mg, 0.375mmol), reacted at 110 degrees for 24 hours; after cooling, under argon, Continue to add palladium acetate (9.2mg, 0.041mmol), tris(4-bromophenyl)amine (482.0mg, 1mmol), piperazine (150.8mg, 1.75mmol) and sodium tert-butoxide (672mg, 7mmol), and then add 30mL of toluene solution was reacted at 120°C for 24 hours; after cooling, it was washed with water and ethanol three times, and dried in a vacuum oven at 50°C for 24 hours to obtain catalyst C1 (0.7% palladium loading).
Embodiment example 2
[0022] Under argon, palladium acetate (1.2 mg, 0.005 mmol) and DIPPF (3.2 mg, 0.0075 mmol) were added to a Schlinker tube containing 2 mL of toluene, stirred for 5 min, and tris(4-bromophenyl)amine was added (120.5mg, 0.25mmol), two-1-adamantylphosphine (75.6mg, 0.25mmol) and sodium tert-butoxide (36mg, 0.375mmol), reacted at 110 degrees for 20 hours; after cooling, under argon, Continue to add palladium acetate (18.4mg, 0.082mmol), tris(4-bromophenyl)amine (482.0mg, 1mmol), piperazine (150.8mg, 1.75mmol) and sodium tert-butoxide (672mg, 7mmol), and then add 30 mL of toluene solution was reacted at 120°C for 24 hours; after cooling, it was washed three times with water and ethanol respectively, and dried in a vacuum oven at 30°C for 20 hours to obtain catalyst C2 (with a palladium loading of 2%).
Embodiment example 3
[0024] Under argon, palladium acetate (1.2 mg, 0.005 mmol) and DIPPF (3.2 mg, 0.0075 mmol) were added to a Schlinker tube containing 2 mL of toluene, stirred for 5 min, and tris(4-bromophenyl)amine was added (120.5mg, 0.25mmol), two-1-adamantylphosphine (75.6mg, 0.25mmol) and sodium tert-butoxide (36mg, 0.375mmol), reacted at 110 degrees for 22 hours; after cooling, under argon, Continue to add palladium acetate (4.6mg, 0.021mmol), tris(4-bromophenyl)amine (482.0mg, 1mmol), piperazine (150.8mg, 1.75mmol) and sodium tert-butoxide (672mg, 7mmol), and then add 30mL of toluene solution was reacted at 120°C for 24 hours; after cooling, it was washed three times with water and ethanol respectively, and dried in a vacuum oven at 40°C for 22 hours to obtain catalyst C3 (0.2% palladium loading).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com