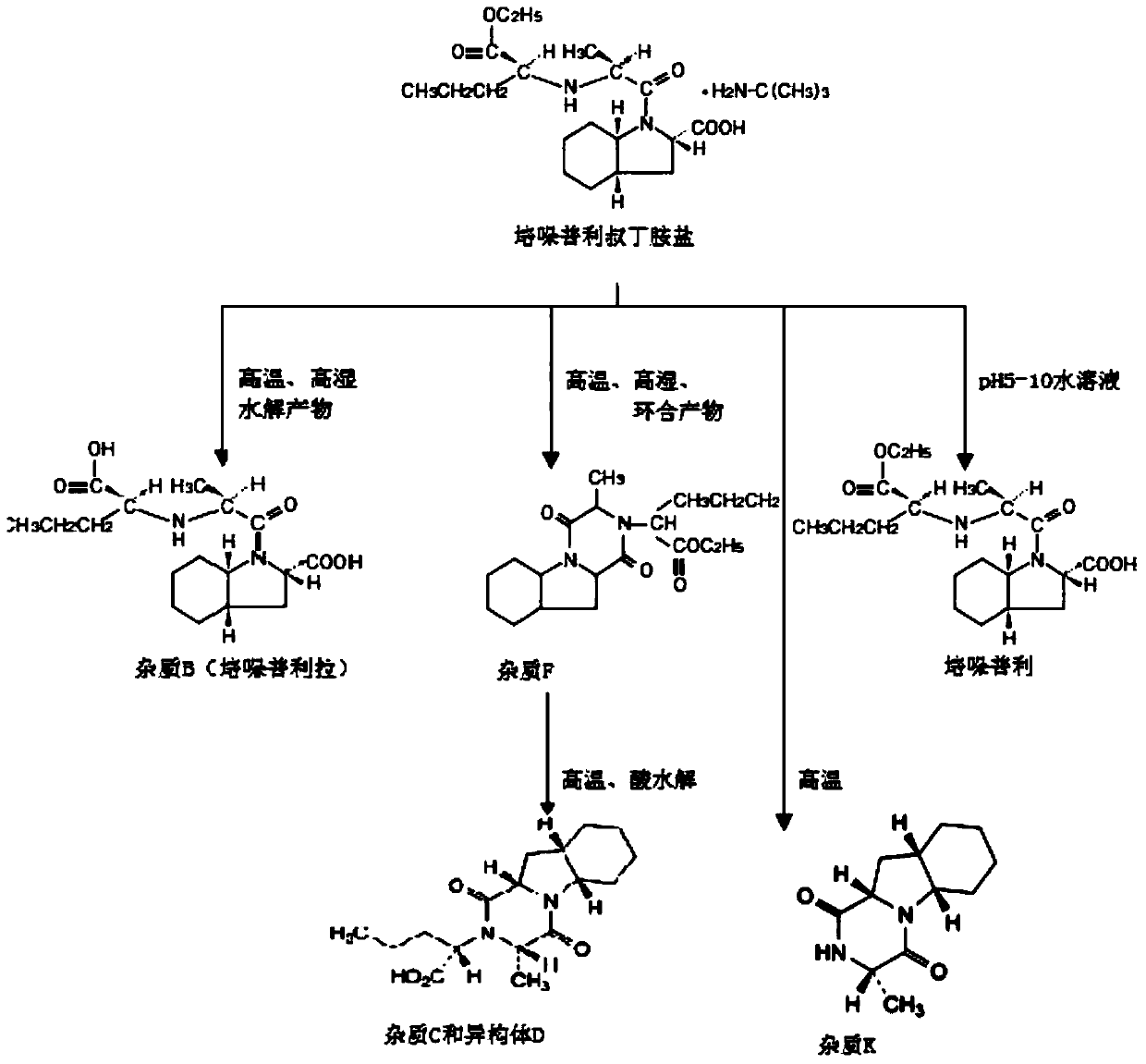
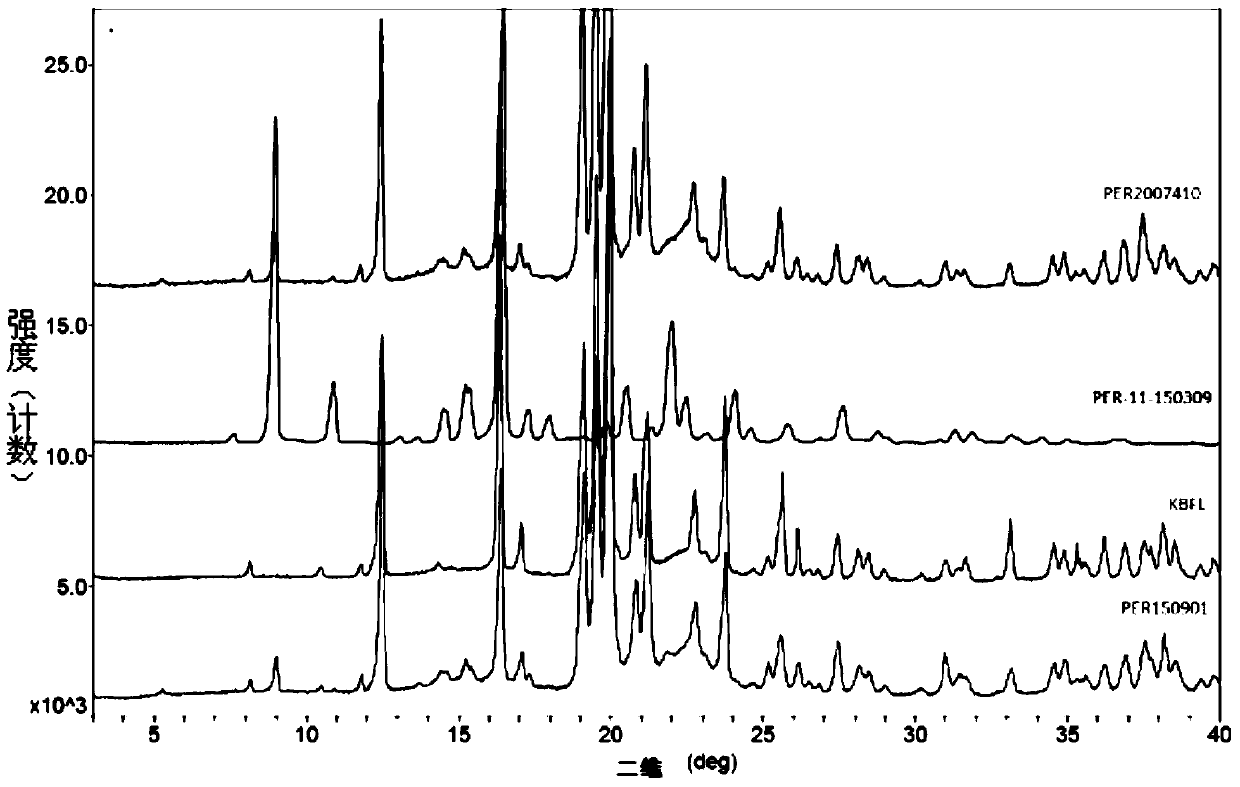
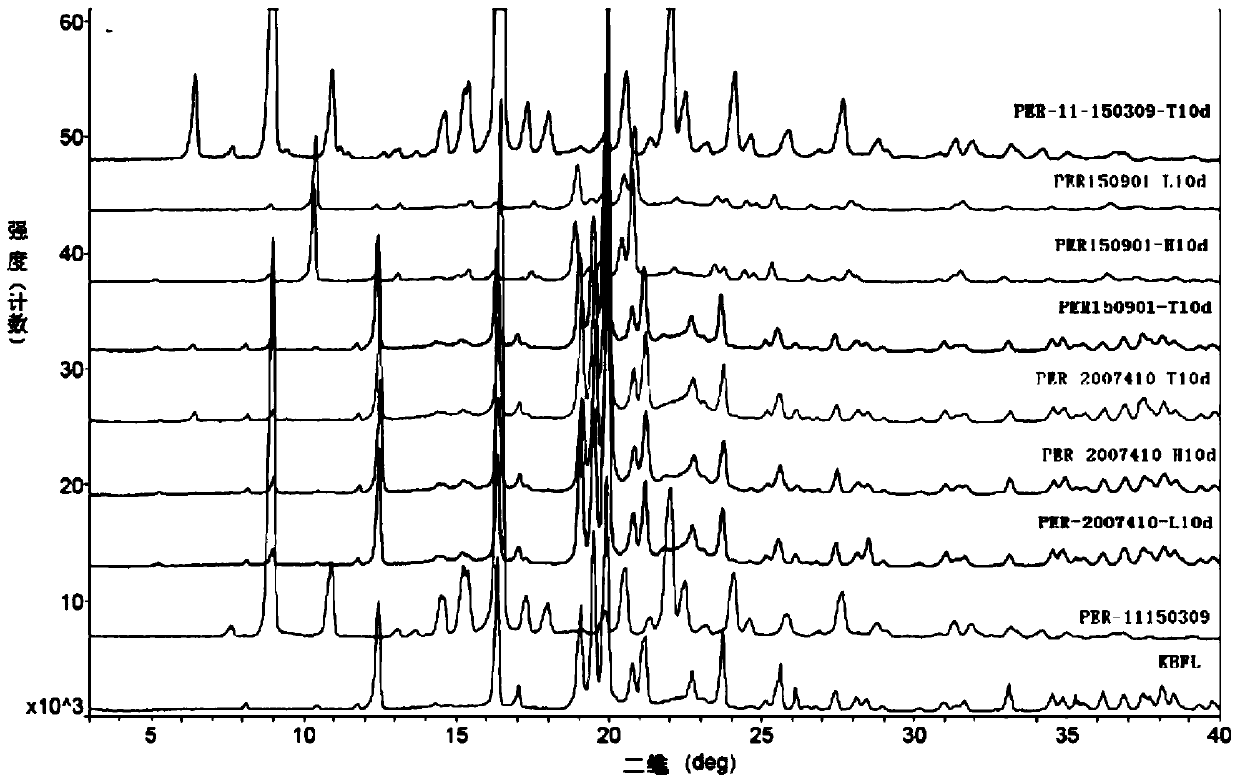
A kind of stable α crystal form perindopril tert-butylamine tablet and its preparation method
A technology of tert-butylamine tablets and perindopril, which is applied in the field of medicine, can solve the problems of impurity degradation rate and difficult crystal form transformation, and achieve the effect of reducing water control and disintegration risks, using fewer types of excipients, and facilitating stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] A kind of perindopril tablet, described perindopril tablet is made up of following components according to parts by weight:
[0039] Alpha crystal form perindopril tert-butylamine: 4 parts;
[0040] Lactose: 65 parts;
[0041] Microcrystalline cellulose: 20 parts;
[0042] Magnesium stearate: 1 part.
[0043] The tablet weighing about 90mg of last system, its preparation process comprises the following steps:
[0044] (1) Take lactose and microcrystalline cellulose and pass through a 50-mesh sieve respectively, mix, dry until the moisture is lower than 4.5%, divide into 3 parts, and control the humidity in the process to be lower than 40% RH.
[0045] (2) Take one portion and mix it with perindopril tert-butylamine passing through a 80-mesh sieve, and pass through a 60-mesh sieve to mix;
[0046] (3) Continue to add a part of the mixed auxiliary materials in (1) and mix;
[0047] (4) Mix (3) with the remaining auxiliary materials in (1) for 20 minutes;
[0048] (5) ...
Embodiment 2
[0054] Embodiment 2: a kind of perindopril tablet, described perindopril tablet is made up of following components according to parts by weight:
[0055] Alpha crystal form perindopril tert-butylamine: 4 parts;
[0056] Lactose: 60 parts;
[0057] Microcrystalline cellulose: 25 parts;
[0058] Magnesium stearate: 1 part.
[0059] The tablet weighing about 90mg of last system, its preparation process comprises the following steps:
[0060] (1) Take lactose and microcrystalline cellulose and pass through a 50-mesh sieve respectively, mix, dry until the moisture is lower than 4.5%, divide into 3 parts, and control the humidity in the process to be lower than 40% RH;
[0061] (2) Take one portion and mix it with perindopril tert-butylamine through a 60-mesh sieve, and mix evenly through a 60-mesh sieve;
[0062] (3) Continue to add a part of the mixed auxiliary materials in (1) and mix;
[0063] (4) Mix (3) with the remaining auxiliary materials in (1) for 20 minutes;
[006...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com