High potency pancreatin pharmaceutical compositions
A technology of composition and pancreatin, which is applied in the directions of drug combination, pancreatin, medical preparations containing active ingredients, etc., can solve the problem of no separate purification and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
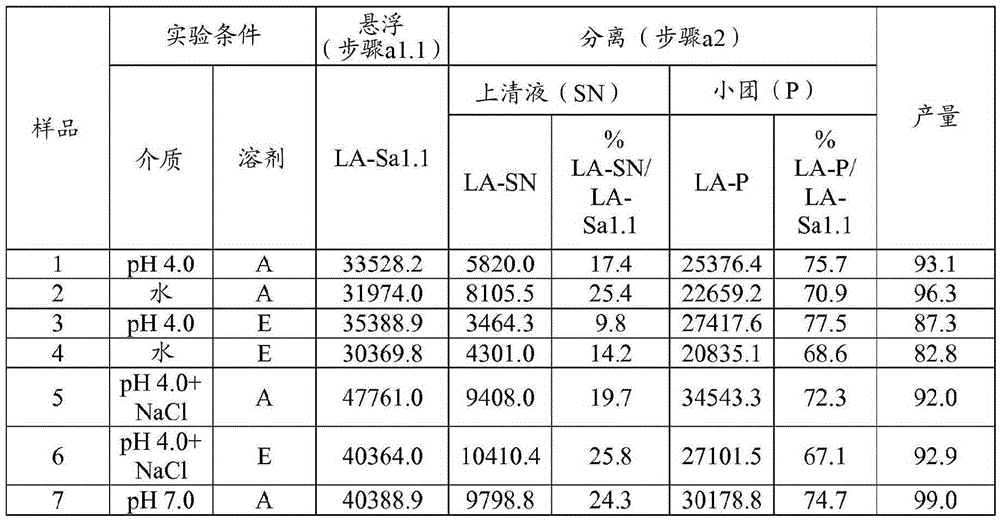
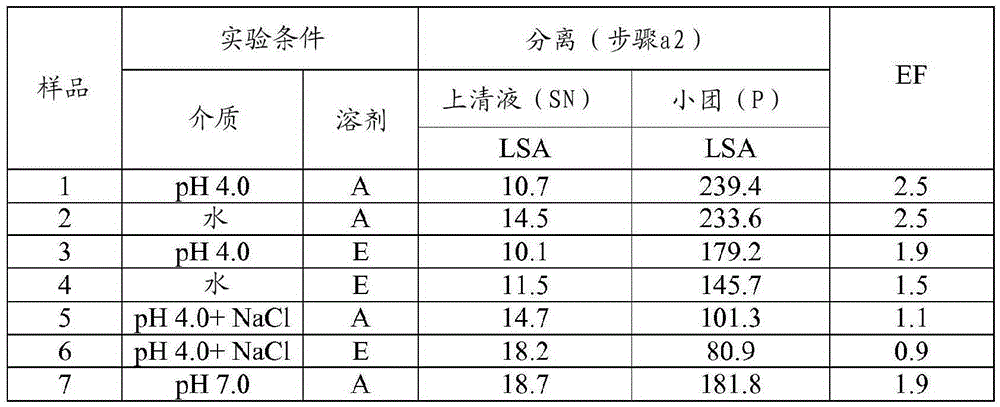
[0093] Example 1 Preparation of HA trypsin; 0.1g / mL; multi-step: suspension, separation, precipitation; (SP=38: acetone: aqueous solvent = 35:65; ethanol: solvent aqueous solvent = 45:55)
[0094] Step a1.1 - Suspension: The starting pancrelipase was dispersed in an aqueous solvent at a concentration of 0.1 g / mL at 4°C and kept under stirring for 30 minutes. Experiments were performed at laboratory scale using 650 mg (when the organic solvent was acetone) or 550 mg (when the organic solvent was ethanol) of starting pancreatic lipase. Four different aqueous solvents were tested for pancrelipase suspension: 1) pH=4.0 buffer (10 mM acetate buffer); 2) pH=7.0 buffer (10 mM phosphate); 3) deionized water (DW); 4) pH = 4.0 buffer (10 mM acetate) containing NaCl (0.5M).
[0095] Step a1.2 - Separation: The suspension from step a1.1 was centrifuged (10 min, 4°C, about 11,000 g) and the supernatant (SN) was separated from the pellet.
[0096] Step a1.3 - Precipitation: An organic s...
Embodiment 2
[0116] Example 2 Preparation of HA trypsin; 0.3g / mL; multi-step: suspension, separation, precipitation (SP=38: acetone: aqueous solvent = 35:65; ethanol: aqueous solvent = 45:55)
[0117] Step a1.1 - Suspension: Pancrelipase (API) was dispersed in an aqueous solvent at a concentration of 0.3 g / mL at 4°C and stirred for 30 minutes. Experiments were performed at laboratory scale using 650 mg (when the organic solvent was acetone) or 550 mg (when the organic solvent was ethanol) of starting pancreatic lipase. Two experiments were each run in different aqueous solvents: 1) pH=4.0 buffer (10 mM acetate buffer); 2) pH=7.0 buffer (10 mM phosphate).
[0118] Step a1.2 - Isolation: The suspension from step a1 was centrifuged (10 min, 4°C, about 11,000 g) and the supernatant (SN) was separated from the pellet.
[0119] Step a1.3 - Precipitation: An organic solvent was added to the supernatant of step a1.2 and the mixture was kept at rest at 4°C for 15 minutes. Described organic solv...
Embodiment 3
[0139] Example 3 Preparation of HA-trypsin; 0.1g / mL; two steps: suspension, precipitation (SP=38: acetone: aqueous solvent = 35:65; ethanol: aqueous solvent = 45:55, where SP (acetone) = 20.2, SP (Ethanol) = 26.0, SP (buffer) = 47.9)
[0140] Step a1.1 - Suspension: Pancrelipase was dispersed in an aqueous solvent at a concentration of 0.1 g / mL at 4°C and kept under stirring for 30 minutes. Experiments were performed at laboratory scale using 650 mg (when the organic solvent was acetone) or 550 mg (when the organic solvent was ethanol) of starting pancreatic lipase. Two experiments were each run in different aqueous solvents: 1) pH=4.0, 10 mM acetate buffer; 2) pH=7.0, 10 mM phosphate buffer.
[0141]Step a1.2 - Precipitation: An organic solvent is added to the suspension of step a1.1 and this mixture is kept at 4°C for 15 minutes. Described organic solvent is acetone or ethanol. Acetone was added in an amount of 35 parts (volume) per 65 parts (volume) of aqueous solvent....
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com