Iodine-containing polysaccharide as well as synthetic method and application thereof
A synthesis method and polysaccharide technology, which are applied in the fields of pharmaceutical formulations, preparations for in vivo tests, X-ray contrast agent preparation, etc., can solve the problems of low iodine content in cellulose acetate, low iodine content, low theoretical iodine content, etc., and achieve Good biocompatibility, high iodine content, and wide range of sources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 10
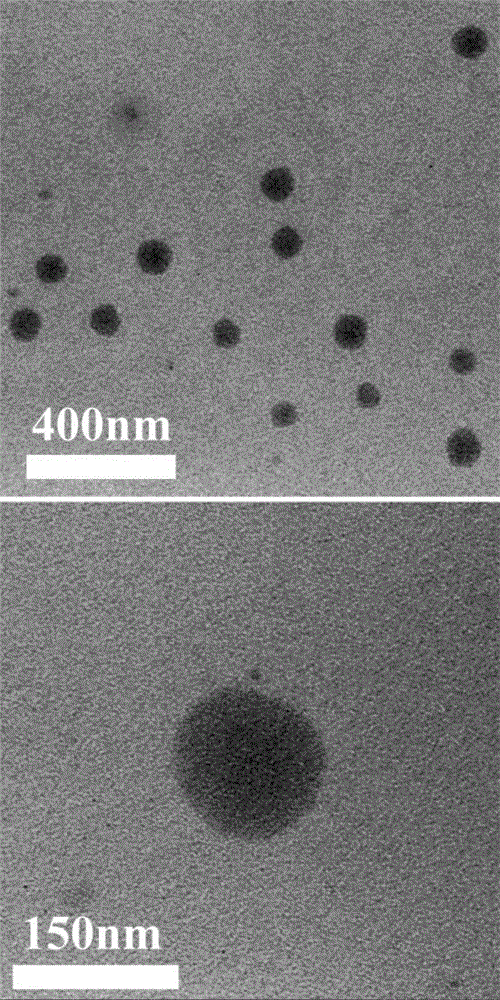
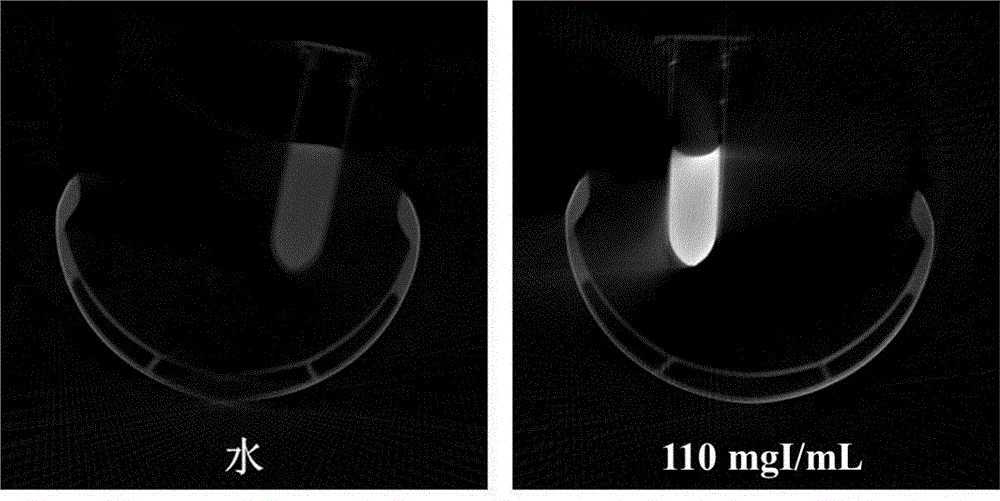
[0066] Example 10 is a specific implementation method of using Micro-CT to test the contrast ability of the iodine-containing nanoparticle CT contrast agent in vitro.
Embodiment 1
[0067] Example 1 (dextran 2,3,5-triiodobenzoate-acyl chloride esterification method)
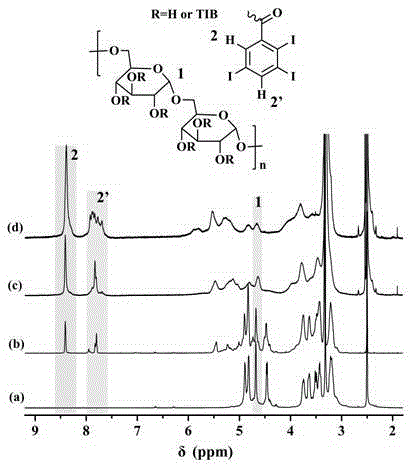
[0068] Dextran 2,3,5-triiodobenzoate, its general molecular structure is as follows:
[0069]
[0070] Wherein, R is selected from hydrogen or 2,3,5-triiodobenzoyl, and R is not all hydrogen, and the degree of grafting of 2,3,5-triiodobenzoyl is 8~300 (based on 100 sugars ring units).
[0071] The above-mentioned iodine-containing polysaccharide was synthesized by the following steps: adding dextran (Dextran, 2g, 12.3mmolAGU) and lithium chloride (2g) into 50mL DMAc under nitrogen protection, heating to 90°C to dissolve completely, and cooling; Add catalytic amount of DMAP, acid-binding agent triethylamine (equivalent to 2,3,5-triiodobenzoyl chloride) and different amounts of 2,3,5-triiodobenzoyl chloride (1.23~49.2mmol, That is, the molar ratio of 2,3,5-triiodobenzoyl chloride to dextran AGU is 0.1~4) DMF solution 50mL, react at 70°C for 1 hour, precipitate in methanol, dissolve with D...
Embodiment 2
[0073] Embodiment 2 (dextran 2,4,6-triiodobenzoate DCC method)
[0074] Dextran 2,4,6-triiodobenzoate, its general molecular structure is as follows:
[0075]
[0076] Wherein, R is selected from hydrogen or 2,4,6-triiodobenzoyl, and R is not all hydrogen, and the degree of grafting of 2,3,5-triiodobenzoyl is 8~300 (based on 100 sugars ring units).
[0077] The above-mentioned iodine-containing polysaccharide was synthesized by the following steps: Add dextran (Dextran, 2g, 12.3mmolAGU) and lithium bromide (2g) into 50mL DMAc under nitrogen protection, heat to 90°C to dissolve completely, cool; add catalyst Add DMAP and different amounts of 2,4,6-triiodobenzoic acid (1.23~49.2mmol, that is, the molar ratio of 2,4,6-triiodobenzoic acid to dextran AGU is 0.1~4) DCC (equivalent to 2,4,6-triiodobenzoic acid), stirred and reacted at room temperature, filtered and precipitated with methanol, purified to obtain iodine-containing polysaccharide. After testing, the degree of gr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com