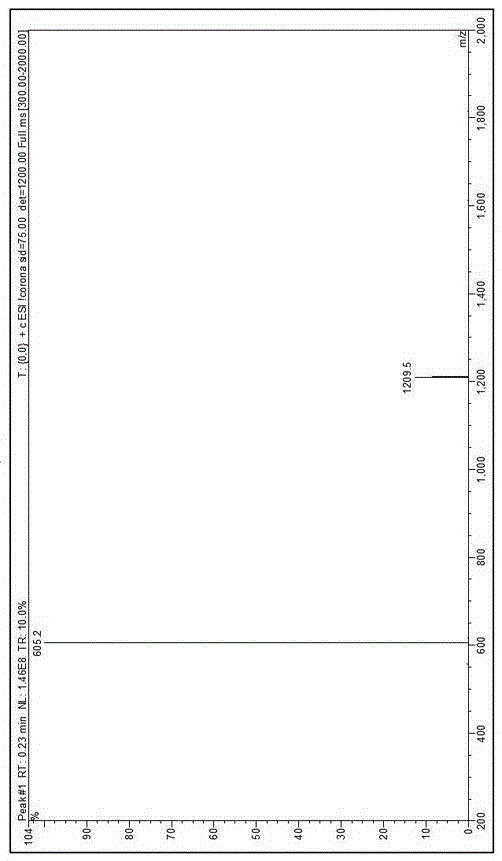
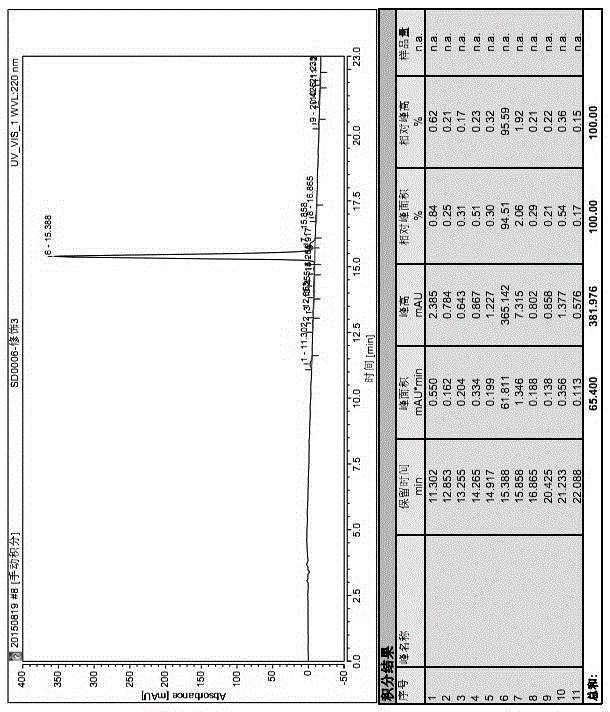
Leuprorelin synthesis method
A technology of leuprolide and synthesis method, which is applied in the preparation method of peptide, chemical instrument and method, organic chemistry and other directions, and can solve problems such as reducing yield and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1, a synthetic method of leuprolide: the method adopts liquid-phase synthesis of a dipeptide intermediate and then uses it in solid-phase synthesis to obtain a fully protected nonapeptide resin, and the fully protected nonapeptide is cut off from the resin in the liquid phase In the phase, ethylamine is connected to obtain fully protected leuprolide, and the crude product of leuprolide is obtained through cracking;
[0037] The specific steps are as follows:
[0038] (1) Fmoc-Leu-OSU fat is obtained by condensing Fmoc-Leu-OH and HOSU under the action of a condensing agent;
[0039] (2) Reaction of Fmoc-Leu-OSU lipid with H-Arg(pbf)-OH under the action of alkali to obtain Fmoc-Leu-Arg(pbf)-OH;
[0040] (3) Fmoc-Pro-OH reacts with CTCResin under the action of alkali to obtain Fmoc-Pro-CTCResin;
[0041] (4) Remove Fmoc, and under the action of a condensing agent, sequentially couple the following amino acids: Fmoc-Leu-Arg(pbf)-OH, Fmoc-D-Leu-OH, Fmoc-Tyr(tBu)-OH...
Embodiment 2
[0044]Example 2, in a synthesis method of leuprolide described in Example 1: the condensing agent described in step (1) is selected from any one of DCC, DIC, and EDC·HCl.
Embodiment 3
[0045] Example 3, in a synthetic method of leuprolide described in Example 1 or 2: the base described in step (2) is selected from any one of DIEA, NMM, Py, DMAP, NaOH, NaHCO3, Na2CO3 kind.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com