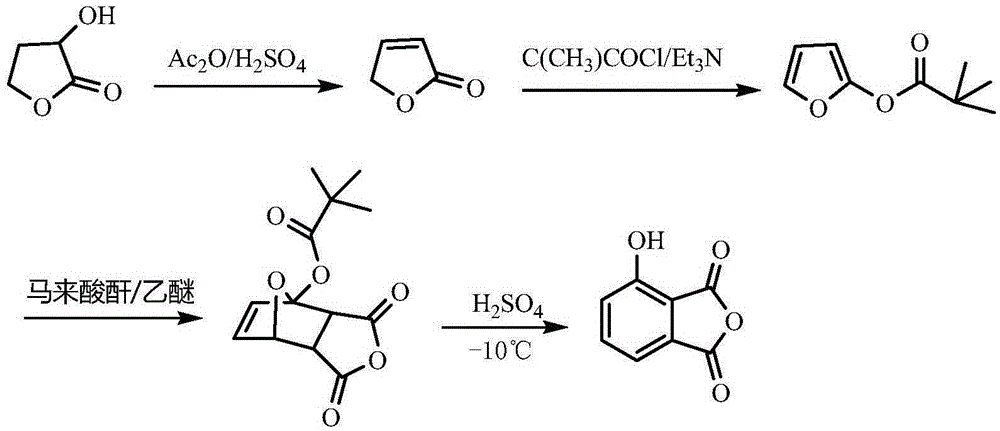
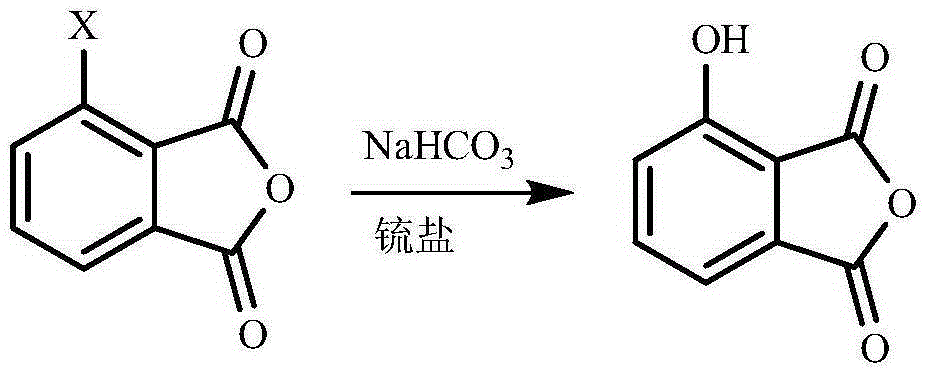
One-pot method for preparing 3-hydroxyphtalic anhydride
A technology of hydroxyphthalic anhydride and compounds, which is applied in the field of organic chemical synthesis, can solve the problems of complex process steps, difficult acquisition of raw materials, and low productivity, and achieve low equipment requirements, easy large-scale preparation, and mild conditions Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Add 3-fluorophthalic acid (184.0g, 1.0mol) and N,N-dimethylformamide (600mL) in the three-necked flask, and start stirring; continue to add potassium hydroxide (112.2g, 2.0mol ) and cuprous iodide (5.7g, 0.03mol), the temperature was raised to 100°C for 6 hours of heat preservation reaction; TLC monitoring (methanol: dichloromethane=1:1, V / V) until the reaction was complete, cooled to room temperature, filtered ; The hydrochloric acid of 6.0mol / L of the filtrate is used to adjust the pH value between 1.0~2.0, and the filtrate is transferred to the there-necked flask, and under the situation of stirring, dicyclohexylcarbodiimide (722.2g, 3.5mol) is added and heated to Reaction at 100°C for 2 hours; TLC monitoring (methanol: dichloromethane = 1:1, V / V) until the reaction is complete, then lower to room temperature; extract with acetone (600mL×3), combine the organic phases, and wash with anhydrous sulfuric acid Dried over magnesium and filtered to obtain 162.3 g of off-wh...
Embodiment 2
[0029] Add 3-fluorophthalic acid (184.0g, 1.0mol) and cyclohexane (1100mL) in the three-necked flask, and start stirring; continue to add potassium tert-butoxide (336.6g, 3.0mol) and trifluoromethane during the reaction Copper sulfonate (18.1g, 0.05mol), heat up to 110°C and keep warm for 4 hours; TLC monitoring (methanol:dichloromethane=1:1, V / V) until the reaction is complete, cool down to room temperature, filter; filtrate Use 6.0mol / L hydrochloric acid to adjust the pH value between 1.0 and 2.0, transfer the filtrate to a three-neck flask, add dicyclohexylcarbodiimide (206.3g, 1.0mol) while stirring, and heat to 120°C Insulated reaction for 3.5 hours; TLC monitoring (methanol: dichloromethane = 1:1, V / V) until the reaction is complete, lowered to room temperature; extracted with methyl tert-butyl ether (1100mL × 3), combined organic phase, and used Dry over anhydrous magnesium sulfate and filter to obtain 154.4 g of 3-hydroxyphthalic anhydride off-white solid with a conver...
Embodiment 3
[0032] Add ethyl 3-fluorophthalate (240.0g, 1.0mol) and dimethyl sulfoxide (1000mL) in the there-necked flask, and start stirring; continue to add potassium tert-butoxide (336.6g, 3.0mol) in the reaction And cuprous iodide (2.0g, 0.01mol), heat up to 120°C and keep warm for 4 hours; TLC monitoring (methanol:dichloromethane=1:1, V / V) until the reaction is complete, cool down to room temperature, and filter; The filtrate was adjusted to a pH value between 1.0 and 2.0 with 6.0mol / L hydrochloric acid, and the filtrate was transferred to a three-necked flask. While stirring, dicyclohexylcarbodiimide (412.7g, 2.0mol) was added and heated to 130 ℃ for 2.5 hours; TLC monitoring (methanol: dichloromethane = 1:1, V / V) until the reaction is complete, then lowered to room temperature; extracted with ethyl acetate (1000mL × 3), combined organic phases, washed with anhydrous Dried over magnesium sulfate and filtered to obtain 210.2 g of off-white solid of 3-hydroxyphthalic anhydride, the co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com