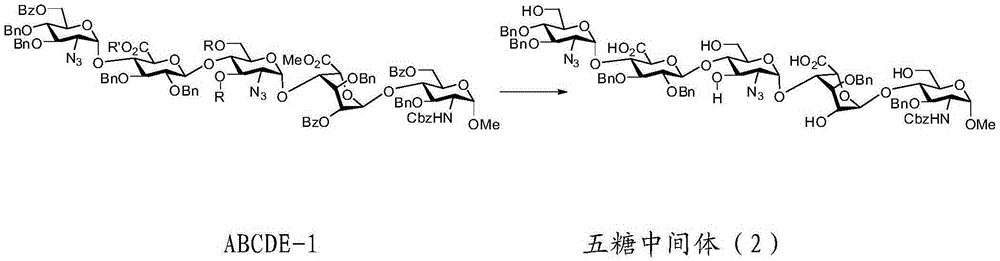
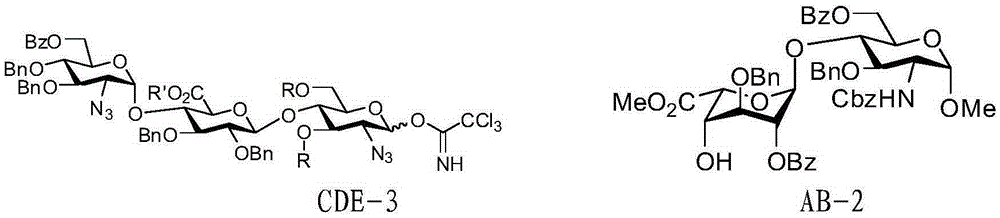
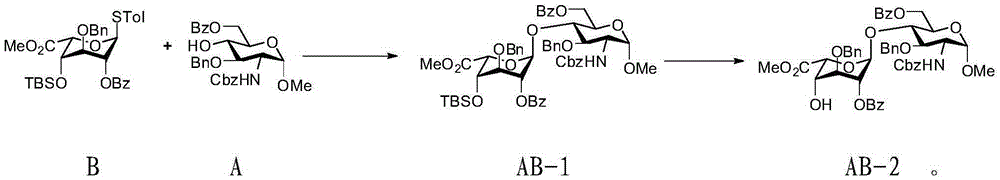
Preparation method for pentosaccharide intermediate of anticoagulant drug fondaparinux sodium
An intermediate and coupling technology, which is applied in the preparation of sugar derivatives, chemical instruments and methods, sugar derivatives, etc., can solve the problems of long synthetic routes, high difficulty, and restrictions on wide application, and achieve convenient and feasible operation and a high preparation route Practical and economical synthesis steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Embodiment 1, the preparation of monosaccharide intermediate B
[0054]
[0055] Compound I (2.4g, 5mmol) was dissolved in a mixed solvent of 60mL dichloromethane and 30mL water, and tetramethylpiperidine nitrogen oxide (156.25mg, 1mmol) and iodobenzene acetate (4.0g, 12.5mmol) were added, Stir at room temperature. After the oxidation is complete, quench the reaction with 20 mL of saturated sodium sulfite aqueous solution, adjust the pH to weak acidity with hydrochloric acid, separate the layers, extract the aqueous phase (20 mL x 4) with dichloromethane, combine the organic phases to dry, and spin to dry the solvent. The product is syrupy.
[0056] After the product obtained in the previous step was pumped on the oil pump for 0.5h, potassium carbonate (1.4g, 10mmol) was added, dissolved in 50mL of dry acetone, and dimethyl sulfate (660uL, 7mmol) was added under ice bath and nitrogen protection conditions, and gradually React at room temperature overnight. After the...
Embodiment 2
[0058] Embodiment 2, the preparation of monosaccharide intermediate C
[0059]
[0060] Dissolve compound III (60g, 188mmol) in toluene (1000mL), add dibutyltin oxide (93.7g, 376mmol), add a water separator, heat slowly to reflux and divide water (about 130°C), and react in this state for 5h Withdraw from the oil bath. Cool down to room temperature, slowly add p-methoxybenzyl chloride (63.8mL, 470mmol) and tetrabutylammonium iodide (34.7g, 94mmol), react at about 90°C for 3h. The reaction solution was lowered to room temperature and left to stand overnight, and tetrabutylammonium iodide was precipitated. The organic phase was washed 3 times with 500 mL water, and the combined aqueous phase was washed twice with ethyl acetate. Combine the organic phases, dry the organic phase with anhydrous sodium sulfate, filter and concentrate to obtain a viscous oily residue, mix the sample with silica gel, and obtain 73 g of light yellow oily product C with a yield of 70% by column chr...
Embodiment 3
[0061] Embodiment 3, the preparation of monosaccharide intermediate D
[0062]
[0063] After compound IV (200mg, 1.0eq) and levulinic acid (4.8mL, 1.1eq) were dissolved in dichloromethane, 4-dimethylaminopyridine (5mg, 0.1eq) and dicyclohexylcarbodiimide (130mg , 2.0eq), react at room temperature, after TLC monitoring is complete, add sodium carbonate under ice bath to neutral pH, add appropriate amount of dichloromethane, wash with saturated brine twice, combine water phase, dichloromethane extract 3 times, combine organic phase, adding anhydrous Na 2 SO 4 Dry and evaporate the solvent. The crude product was recrystallized with petroleum ether / ethyl acetate system to obtain 232 mg of monosaccharide intermediate D with a yield of 96%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com